Evaluation of the core antigen assay as a second-line supplemental test for diagnosis of active hepatitis C virus infection
- PMID: 15364989
- PMCID: PMC516310
- DOI: 10.1128/JCM.42.9.4054-4059.2004
Evaluation of the core antigen assay as a second-line supplemental test for diagnosis of active hepatitis C virus infection
Abstract
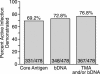
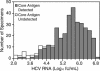
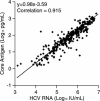
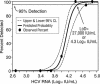
The British Columbia Center for Disease Control laboratory performs approximately 95% of all hepatitis C virus (HCV) antibody tests for the province's 4 million inhabitants. In 2002, the laboratory tested 96,000 specimens for anti-HCV antibodies, of which 4,800 (5%) were seroreactive and required confirmation of active infection. Although HCV RNA assays with a sensitivity of 50 IU/ml or less are recommended for the confirmation of active HCV infection, given the large number of seroreactive specimens tested annually, we evaluated the Ortho trak-C assay (OTCA) as a second-line confirmatory test and determined its limit of detection (LoD). Of 502 specimens from treatment-naïve anti-HCV-positive individuals, 478 had sufficient volumes for evaluation by the OTCA and HCV RNA tests. Core antigen was not detected in 147 of 478 (30.8%) of these specimens, of which 37 of 147 (25.2%) were shown to be viremic by the VERSANT HCV (version 3.0) (branched-DNA) assay and/or the VERSANT HCV qualitative assay. Testing of 144 replicates of a World Health Organization standard dilution series indicated that the LoD of OTCA was approximately 27,000 IU/ml. This LoD is consistent with the inability of OTCA to detect core antigen in clinical specimens with low viral loads. We conclude that OTCA has limited value as a confirmatory test for the diagnosis of active HCV infection because 37 of 367 (10%) of viremic specimens had undetectable core antigen. Qualitative HCV RNA testing remains the present standard for the confirmation of active HCV infection in the diagnostic setting.
Figures
Similar articles
-
Evaluation of a total hepatitis C virus (HCV) core antigen assay for the detection of antigenaemia in anti-HCV positive individuals.J Med Virol. 2004 Jul;73(3):397-403. doi: 10.1002/jmv.20105. J Med Virol. 2004. PMID: 15170635
-
Usefulness of the hepatitis C virus core antigen assay for screening of a population undergoing routine medical checkup.J Clin Microbiol. 2005 Apr;43(4):1722-6. doi: 10.1128/JCM.43.4.1722-1726.2005. J Clin Microbiol. 2005. PMID: 15814991 Free PMC article.
-
Evaluation of a total core antigen assay for the diagnosis of hepatitis C virus infection in hemodialysis patients.J Med Virol. 2005 Dec;77(4):502-8. doi: 10.1002/jmv.20485. J Med Virol. 2005. PMID: 16254976
-
Diagnostic tests for hepatitis C.Hepatology. 1997 Sep;26(3 Suppl 1):43S-47S. doi: 10.1002/hep.510260708. Hepatology. 1997. PMID: 9305663 Review.
-
The role of core antigen detection in management of hepatitis C: a critical review.J Clin Virol. 2005 Feb;32(2):92-101. doi: 10.1016/j.jcv.2004.10.005. J Clin Virol. 2005. PMID: 15653411 Review.
Cited by
-
Factors associated with spontaneous clearance of hepatitis C virus among illicit drug users.Can J Gastroenterol. 2007 Jul;21(7):447-51. doi: 10.1155/2007/796325. Can J Gastroenterol. 2007. PMID: 17637948 Free PMC article.
-
Evaluation of an antigen-antibody "combination" enzyme linked immunosorbent assay for diagnosis of hepatitis C virus infections.Ethiop J Health Sci. 2014 Oct;24(4):343-52. doi: 10.4314/ejhs.v24i4.10. Ethiop J Health Sci. 2014. PMID: 25489199 Free PMC article.
-
Early detection of hepatitis C virus infection by use of a new combined antigen-antibody detection assay: potential use for high-risk individuals.J Clin Microbiol. 2006 Apr;44(4):1561-3. doi: 10.1128/JCM.44.4.1561-1563.2006. J Clin Microbiol. 2006. PMID: 16597894 Free PMC article.
-
Health care costs associated with hepatitis C: a longitudinal cohort study.Can J Gastroenterol. 2010 Dec;24(12):717-26. doi: 10.1155/2010/569692. Can J Gastroenterol. 2010. PMID: 21165379 Free PMC article.
-
Simultaneous detection of hepatitis C virus (HCV) core antigen and anti-HCV antibodies improves the early detection of HCV infection.J Clin Microbiol. 2005 Aug;43(8):3877-83. doi: 10.1128/JCM.43.8.3877-3883.2005. J Clin Microbiol. 2005. PMID: 16081925 Free PMC article.
References
-
- Alter, M. J., D. Kruszon-Moran, O. V. Nainan, G. M. McQuillan, F. Gao, L. A. Moyer, R. A. Kaslow, and H. S. Margolis. 1999. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N. Engl. J. Med. 341:556-562. - PubMed
-
- Alter, M. J., W. L. Kuhnert, and L. Finelli. 2003. Guidelines for laboratory testing and result reporting of antibody to hepatitis C virus. Morb. Mortal. Wkly. Rep. Recomm. Rep. 52:1-13, 15. - PubMed
-
- Armstrong, G. L. 2003. Commentary: modeling the epidemiology of hepatitis C and its complications. Int. J. Epidemiol. 32:725-726. - PubMed
-
- Barrett, S., N. Kieran, E. Ryan, J. C. O'Keane, and J. Crowe. 2001. Intrahepatic hepatitis C viral RNA status of serum polymerase chain reaction-negative individuals with histological changes on liver biopsy. Hepatology 33:1496-1502. - PubMed
-
- Beld, M., R. Sentjens, S. Rebers, C. Weegink, J. Weel, C. Sol, and R. Boom. 2002. Performance of the new Bayer VERSANT HCV RNA 3.0 assay for quantitation of hepatitis C virus RNA in plasma and serum: conversion to international units and comparison with the Roche COBAS Amplicor HCV Monitor, version 2.0, assay. J. Clin. Microbiol. 40:788-793. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

