Field evaluation of a rapid human immunodeficiency virus (HIV) serial serologic testing algorithm for diagnosis and differentiation of HIV type 1 (HIV-1), HIV-2, and dual HIV-1-HIV-2 infections in West African pregnant women
- PMID: 15365003
- PMCID: PMC516348
- DOI: 10.1128/JCM.42.9.4147-4153.2004
Field evaluation of a rapid human immunodeficiency virus (HIV) serial serologic testing algorithm for diagnosis and differentiation of HIV type 1 (HIV-1), HIV-2, and dual HIV-1-HIV-2 infections in West African pregnant women
Abstract
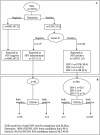
We evaluated a two-rapid-test serial algorithm using the Determine and Genie II rapid assays, performed on-site in four peripheral laboratories during the French Agence Nationale de Recherches sur le SIDA (ANRS) 1201/1202 Ditrame Plus cohort developed for prevention of mother-to-child transmission of human immunodeficiency virus (HIV) infection in Côte d'Ivoire. A total of 1,039 specimens were retested by two commercial enzyme-linked immunosorbent assays (ELISAs). The following specimens were tested: 315 specimens found on-site to be infected with HIV type 1 (HIV-1), 8 specimens found on-site to be infected with HIV-2, 71 specimens found on-site to be infected with both HIV-1 and HIV-2, 40 specimens found on-site to have indeterminate results for HIV infection, and 605 specimens taken during a quality assurance program. For HIV discrimination, 99 positive serum samples (20 with HIV-1, 8 with HIV-2, and 71 with HIV-1 and HIV-2 on the basis of our rapid test algorithm) were retested by the Peptilav test, Western blot (WB) assays, and homemade monospecific ELISAs. Real-time DNA PCRs for the detection of HIV-1 and HIV-2 were performed with peripheral blood mononuclear cells from 35 women diagnosed on-site with HIV-1 and HIV-2 infections. Compared to the results of the ELISAs, the sensitivities of the Determine and Genie II assays were 100% (95% lower limit [95% LL], 99.1%) and 99.5% (95% confidence interval [95% CI], 98.2 to 99.9%), respectively. The specificities were 98.4% (95% CI, 96.9 to 99.3%) and 100% (95% LL, 99.3%), respectively. All serological assays gave concordant results for infections with single types. By contrast, for samples found to be infected with dual HIV types by the Genie II assay, dual reactivity was detected for only 37 samples (52.1%) by WB assays, 34 samples (47.9%) by the Peptilav assay, and 23 samples (32.4%) by the monospecific ELISAs. For specimens with dual reactivity by the Genie II assay, the rates of concordance between the real-time PCR assays and the serological assays were 25.7% for the Genie II assay, 82.9% for the Peptilav assay, 74.3% for WB assays, and 80% for the homemade ELISAs. Our algorithm provided high degrees of sensitivity and specificity comparable to those of ELISAs. Even if they are rare, women identified by the Genie II assay as being infected with HIV-1 and HIV-2 mostly appeared to be infected only with HIV-2.
Figures
Similar articles
-
Sensitivity and specificity of human immunodeficiency virus rapid serologic assays and testing algorithms in an antenatal clinic in Abidjan, Ivory Coast.J Clin Microbiol. 2001 May;39(5):1808-12. doi: 10.1128/JCM.39.5.1808-1812.2001. J Clin Microbiol. 2001. PMID: 11325995 Free PMC article.
-
Rapid and simple screening and supplemental testing for HIV-1 and HIV-2 infections in west Africa.AIDS. 1993 Jun;7(6):883-5. doi: 10.1097/00002030-199306000-00019. AIDS. 1993. PMID: 8395857
-
Field evaluation of a combination of monospecific enzyme-linked immunosorbent assays for type-specific diagnosis of human immunodeficiency virus type 1 (HIV-1) and HIV-2 infections in HIV-seropositive persons in Abidjan, Ivory Coast.J Clin Microbiol. 1998 Jan;36(1):123-7. doi: 10.1128/JCM.36.1.123-127.1998. J Clin Microbiol. 1998. PMID: 9431934 Free PMC article.
-
Evaluation of HIV serial and parallel serologic testing algorithms in Abidjan, Côte d'Ivoire.AIDS. 1999 Jan 14;13(1):109-17. doi: 10.1097/00002030-199901140-00015. AIDS. 1999. PMID: 10207552
-
Field evaluation of alternative testing strategies for diagnosis and differentiation of HIV-1 and HIV-2 infections in an HIV-1 and HIV-2-prevalent area.AIDS. 1997 Dec;11(15):1815-22. doi: 10.1097/00002030-199715000-00005. AIDS. 1997. PMID: 9412699
Cited by
-
New sensitive one-step real-time duplex PCR method for group A and B HIV-2 RNA load.J Clin Microbiol. 2014 Aug;52(8):3017-22. doi: 10.1128/JCM.00724-14. Epub 2014 Jun 11. J Clin Microbiol. 2014. PMID: 24920771 Free PMC article.
-
Clinical diagnostic accuracy of the HIV rapid test ACCU-TELL anti-HIV 1 + 2 in sub-Saharan African region.BMC Infect Dis. 2025 Jul 1;25(1):812. doi: 10.1186/s12879-025-10496-3. BMC Infect Dis. 2025. PMID: 40597687 Free PMC article.
-
Response to "Rapid tests for HIV type discrimination in West Africa may perform differently".J Int AIDS Soc. 2015 Jan 29;18(1):19380. doi: 10.7448/IAS.18.1.19380. eCollection 2015. J Int AIDS Soc. 2015. PMID: 25636349 Free PMC article. No abstract available.
-
Characteristics, immunological response & treatment outcomes of HIV-2 compared with HIV-1 & dual infections (HIV 1/2) in Mumbai.Indian J Med Res. 2010 Dec;132(6):683-9. Indian J Med Res. 2010. PMID: 21245615 Free PMC article.
-
Re-testing and misclassification of HIV-2 and HIV-1&2 dually reactive patients among the HIV-2 cohort of the West African Database to evaluate AIDS collaboration.J Int AIDS Soc. 2014 Aug 11;17(1):19064. doi: 10.7448/IAS.17.1.19064. eCollection 2014. J Int AIDS Soc. 2014. PMID: 25128907 Free PMC article.
References
-
- Andersson, S., Z. da Silva, H. Norrgren, F. Dias, and G. Biberfeld. 1997. Field evaluation of alternative testing strategies for diagnosis and differentiation of HIV-1 and HIV-2 infections in an HIV-1 and HIV-2-prevalent area. AIDS 11:1815-1822. - PubMed
-
- Centers for Disease Control and Prevention. 1998. Update: HIV counseling and testing using rapid tests—United States, 1995. Morb. Mortal. Wkly. Rep. 47:211-215. - PubMed
-
- Centers for Disease Control and Prevention. 1990. Update: serologic testing for HIV-1 antibody—United States. Morb. Mortal. Wkly. Rep. 39:69-72. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

