Mechanism of block of hEag1 K+ channels by imipramine and astemizole
- PMID: 15365094
- PMCID: PMC2233905
- DOI: 10.1085/jgp.200409041
Mechanism of block of hEag1 K+ channels by imipramine and astemizole
Abstract
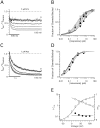
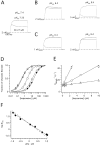
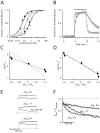
Ether à go-go (Eag; KV10.1) voltage-gated K+ channels have been detected in cancer cell lines of diverse origin and shown to influence their rate of proliferation. The tricyclic antidepressant imipramine and the antihistamine astemizole inhibit the current through Eag1 channels and reduce the proliferation of cancer cells. Here we describe the mechanism by which both drugs block human Eag1 (hEag1) channels. Even if both drugs differ in their affinity for hEag1 channels (IC50s are approximately 2 microM for imipramine and approximately 200 nM for astemizole) and in their blocking kinetics, both drugs permeate the membrane and inhibit the hEag1 current by selectively binding to open channels. Furthermore, both drugs are weak bases and the IC50s depend on both internal an external pH, suggesting that both substances cross the membrane in their uncharged form and act from inside the cell in their charged forms. Accordingly, the block by imipramine is voltage dependent and antagonized by intracellular TEA, consistent with imipramine binding in its charged form to a site located close to the inner end of the selectivity filter. Using inside- and outside-out patch recordings, we found that a permanently charged, quaternary derivative of imipramine (N-methyl-imipramine) only blocks channels from the intracellular side of the membrane. In contrast, the block by astemizole is voltage independent. However, as astemizole competes with imipramine and intracellular TEA for binding to the channel, it is proposed to interact with an overlapping intracellular binding site. The significance of these findings, in the context of structure-function of channels of the eag family is discussed.
Figures

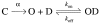






Similar articles
-
Different relevance of inactivation and F468 residue in the mechanisms of hEag1 channel blockage by astemizole, imipramine and dofetilide.FEBS Lett. 2006 Sep 18;580(21):5059-66. doi: 10.1016/j.febslet.2006.08.030. Epub 2006 Aug 28. FEBS Lett. 2006. PMID: 16949586
-
High affinity HERG K(+) channel blockade by the antiarrhythmic agent dronedarone: resistance to mutations of the S6 residues Y652 and F656.Biochem Biophys Res Commun. 2004 Dec 17;325(3):883-91. doi: 10.1016/j.bbrc.2004.10.127. Biochem Biophys Res Commun. 2004. PMID: 15541373
-
Characterization of a hERG screen using the IonWorks HT: comparison to a hERG rubidium efflux screen.Assay Drug Dev Technol. 2005 Feb;3(1):47-57. doi: 10.1089/adt.2005.3.47. Assay Drug Dev Technol. 2005. PMID: 15798395
-
The HERG K+ channel: progress in understanding the molecular basis of its unusual gating kinetics.Eur Biophys J. 2004 Apr;33(2):89-97. doi: 10.1007/s00249-003-0338-3. Epub 2003 Sep 10. Eur Biophys J. 2004. PMID: 13680209 Review.
-
Astemizole: an old anti-histamine as a new promising anti-cancer drug.Anticancer Agents Med Chem. 2011 Mar;11(3):307-14. doi: 10.2174/187152011795347513. Anticancer Agents Med Chem. 2011. PMID: 21443504 Review.
Cited by
-
Potassium Channels in Cancer.Handb Exp Pharmacol. 2021;267:253-275. doi: 10.1007/164_2021_465. Handb Exp Pharmacol. 2021. PMID: 33864122
-
The hEag1 K+ Channel Inhibitor Astemizole Stimulates Ca2+ Deposition in SaOS-2 and MG-63 Osteosarcoma Cultures.Int J Mol Sci. 2022 Sep 11;23(18):10533. doi: 10.3390/ijms231810533. Int J Mol Sci. 2022. PMID: 36142445 Free PMC article.
-
EAG channels expressed in microvillar photoreceptors are unsuited to diurnal vision.J Physiol. 2017 Aug 15;595(16):5465-5479. doi: 10.1113/JP273612. Epub 2017 Feb 22. J Physiol. 2017. PMID: 28087896 Free PMC article.
-
Precision Medicine in Antidepressants Treatment.Handb Exp Pharmacol. 2023;280:131-186. doi: 10.1007/164_2023_654. Handb Exp Pharmacol. 2023. PMID: 37195310 Review.
-
The combination astemizole-gefitinib as a potential therapy for human lung cancer.Onco Targets Ther. 2017 Dec 6;10:5795-5803. doi: 10.2147/OTT.S144506. eCollection 2017. Onco Targets Ther. 2017. PMID: 29263676 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

