Structural basis for the interaction of Escherichia coli NusA with protein N of phage lambda
- PMID: 15365170
- PMCID: PMC518830
- DOI: 10.1073/pnas.0405883101
Structural basis for the interaction of Escherichia coli NusA with protein N of phage lambda
Abstract
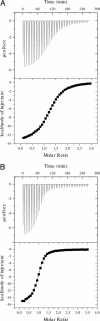
The C terminus of transcription factor NusA from Escherichia coli comprises two repeat units, which bind during antitermination to protein N from phage lambda. To delineate the structural basis of the NusA-lambdaN interaction, we attempted to crystallize the NusA C-terminal repeats in complex with a lambdaN peptide (residues 34-47). The two NusA domains became proteolytically separated during crystallization, and crystals contained two copies of the first repeat unit in contact with a single lambdaN fragment. The NusA modules employ identical regions to contact the peptide but approach the ligand from opposite sides. In contrast to the alpha-helical conformation of the lambdaN N terminus in complex with boxB RNA, residues 34-40 of lambdaN remain extended upon interaction with NusA. Mutational analyses indicated that only one of the observed NusA-lambdaN interaction modes is biologically significant, supporting an equimolar ratio of NusA and lambdaN in antitermination complexes. Solution studies indicated that additional interactions are fostered by the second NusA repeat unit, consistent with known compensatory mutations in NusA and lambdaN. Contrary to the RNA polymerase alpha subunit, lambdaN binding does not stimulate RNA interaction of NusA. The results demonstrate that lambdaN serves as a scaffold to closely oppose NusA and the mRNA in antitermination complexes.
Figures


References
-
- Greenblatt, J., Mah, T. F., Legault, P., Mogridge, J., Li, J. & Kay, L. E. (1998) Cold Spring Harbor Symp. Quant. Biol. 63, 327–336. - PubMed
-
- Nudler, E. & Gottesman, M. E. (2002) Genes Cells 7, 755–768. - PubMed
-
- Mogridge, J., Mah, T. F. & Greenblatt, J. (1995) Genes Dev. 9, 2831–2845. - PubMed
-
- Mogridge, J., Mah, T. F. & Greenblatt, J. (1998) J. Biol. Chem. 273, 4143–4148. - PubMed
-
- Van Gilst, M. R. & von Hippel, P. H. (1997) J. Mol. Biol. 274, 160–173. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

