Mixing active-site components: a recipe for the unique enzymatic activity of a telomere resolvase
- PMID: 15365172
- PMCID: PMC518831
- DOI: 10.1073/pnas.0405762101
Mixing active-site components: a recipe for the unique enzymatic activity of a telomere resolvase
Abstract
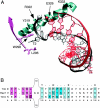
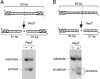
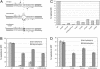
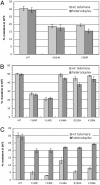
The ResT protein, a telomere resolvase from Borrelia burgdorferi, processes replication intermediates into linear replicons with hairpin ends by using a catalytic mechanism similar to that for tyrosine recombinases and type IB topoisomerases. We have identified in ResT a hairpin binding region typically found in cut-and-paste transposases. We show that substitution of residues within this region results in a decreased ability of these mutants to catalyze telomere resolution. However, the mutants are capable of resolving heteroduplex DNA substrates designed to allow spontaneous destabilization and prehairpin formation. These findings support the existence of a hairpin binding region in ResT, the only known occurrence outside a transposase. The combination of transposase-like and tyrosine-recombinase-like domains found in ResT indicates the use of a composite active site and helps explain the unique breakage-and-reunion reaction observed with this protein. Comparison of the ResT sequence with other known telomere resolvases suggests that a hairpin binding motif is a common feature in this class of enzyme; the sequence motif also appears in the RAG recombinases. Finally, our data support a mechanism of action whereby ResT induces prehairpin formation before the DNA cleavage step.
Figures
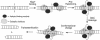
Similar articles
-
Sequence-specific recognition but position-dependent cleavage of two distinct telomeres by the Borrelia burgdorferi telomere resolvase, ResT.Mol Microbiol. 2003 May;48(4):901-11. doi: 10.1046/j.1365-2958.2003.03485.x. Mol Microbiol. 2003. PMID: 12753185
-
Catalytic residues of the telomere resolvase ResT: a pattern similar to, but distinct from, tyrosine recombinases and type IB topoisomerases.J Biol Chem. 2004 Dec 17;279(51):53699-706. doi: 10.1074/jbc.M409001200. Epub 2004 Oct 6. J Biol Chem. 2004. PMID: 15471873
-
Telomere resolution by Borrelia burgdorferi ResT through the collaborative efforts of tethered DNA binding domains.Mol Microbiol. 2007 May;64(3):580-90. doi: 10.1111/j.1365-2958.2007.05691.x. Mol Microbiol. 2007. PMID: 17462009
-
Split target specificity of ResT: a design for protein delivery, site selectivity and regulation of enzyme activity?Mol Microbiol. 2007 May;64(3):575-9. doi: 10.1111/j.1365-2958.2007.05700.x. Mol Microbiol. 2007. PMID: 17462008 Review.
-
Hairpin telomeres and genome plasticity in Borrelia: all mixed up in the end.Mol Microbiol. 2005 Nov;58(3):625-35. doi: 10.1111/j.1365-2958.2005.04872.x. Mol Microbiol. 2005. PMID: 16238614 Review.
Cited by
-
An enzyme-catalyzed multistep DNA refolding mechanism in hairpin telomere formation.PLoS Biol. 2013;11(1):e1001472. doi: 10.1371/journal.pbio.1001472. Epub 2013 Jan 29. PLoS Biol. 2013. PMID: 23382649 Free PMC article.
-
Comparative sequence analysis of IS50/Tn5 transposase.J Bacteriol. 2004 Dec;186(24):8240-7. doi: 10.1128/JB.186.24.8240-8247.2004. J Bacteriol. 2004. PMID: 15576772 Free PMC article.
-
An interlocked dimer of the protelomerase TelK distorts DNA structure for the formation of hairpin telomeres.Mol Cell. 2007 Sep 21;27(6):901-13. doi: 10.1016/j.molcel.2007.07.026. Mol Cell. 2007. PMID: 17889664 Free PMC article.
-
Requirements for DNA hairpin formation by RAG1/2.Proc Natl Acad Sci U S A. 2007 Feb 27;104(9):3078-83. doi: 10.1073/pnas.0611293104. Epub 2007 Feb 16. Proc Natl Acad Sci U S A. 2007. PMID: 17307873 Free PMC article.
-
The telomere resolvase of the Lyme disease spirochete, Borrelia burgdorferi, promotes DNA single-strand annealing and strand exchange.Nucleic Acids Res. 2013 Dec;41(22):10438-48. doi: 10.1093/nar/gkt832. Epub 2013 Sep 17. Nucleic Acids Res. 2013. PMID: 24049070 Free PMC article.
References
-
- Barbour, A. G. (2001) in Emerging Infections 5, eds. Scheld, M. W., Craig, W. A. & Hughes, J. M. (Am. Soc. Microbiol., Washington, DC), pp. 153–173.
-
- Stewart, P., Rosa, P. A. & Tilly, K. (2004) in Plasmid Biology, eds. Phillips, G. & Funnell, B. (Am. Soc. Microbiol., Washington, DC), pp. 291–301.
-
- Chaconas, G. & Chen, C. W. (2004) in The Bacterial Chromosome, ed. Higgins, N. P. (Am. Soc. Microbiol., Washington, DC), in press.
-
- Fraser, C. M., Casjens, S., Huang, W. M., Sutton, G. G., Clayton, R., Lathigra, R., White, O., Ketchum, K. A., Dodson, R., Hickey, E. K., et al. (1997) Nature 390, 580–586. - PubMed
-
- Casjens, S., Palmer, N., van Vugt, R., Huang, W. H., Stevenson, B., Rosa, P., Lathigra, R., Sutton, G., Peterson, J., Dodson, R. J., et al. (2000) Mol. Microbiol. 35, 490–516. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

