Overexpression of TGF-beta by infiltrated granulocytes correlates with the expression of collagen mRNA in pancreatic cancer
- PMID: 15365564
- PMCID: PMC2409911
- DOI: 10.1038/sj.bjc.6602141
Overexpression of TGF-beta by infiltrated granulocytes correlates with the expression of collagen mRNA in pancreatic cancer
Abstract
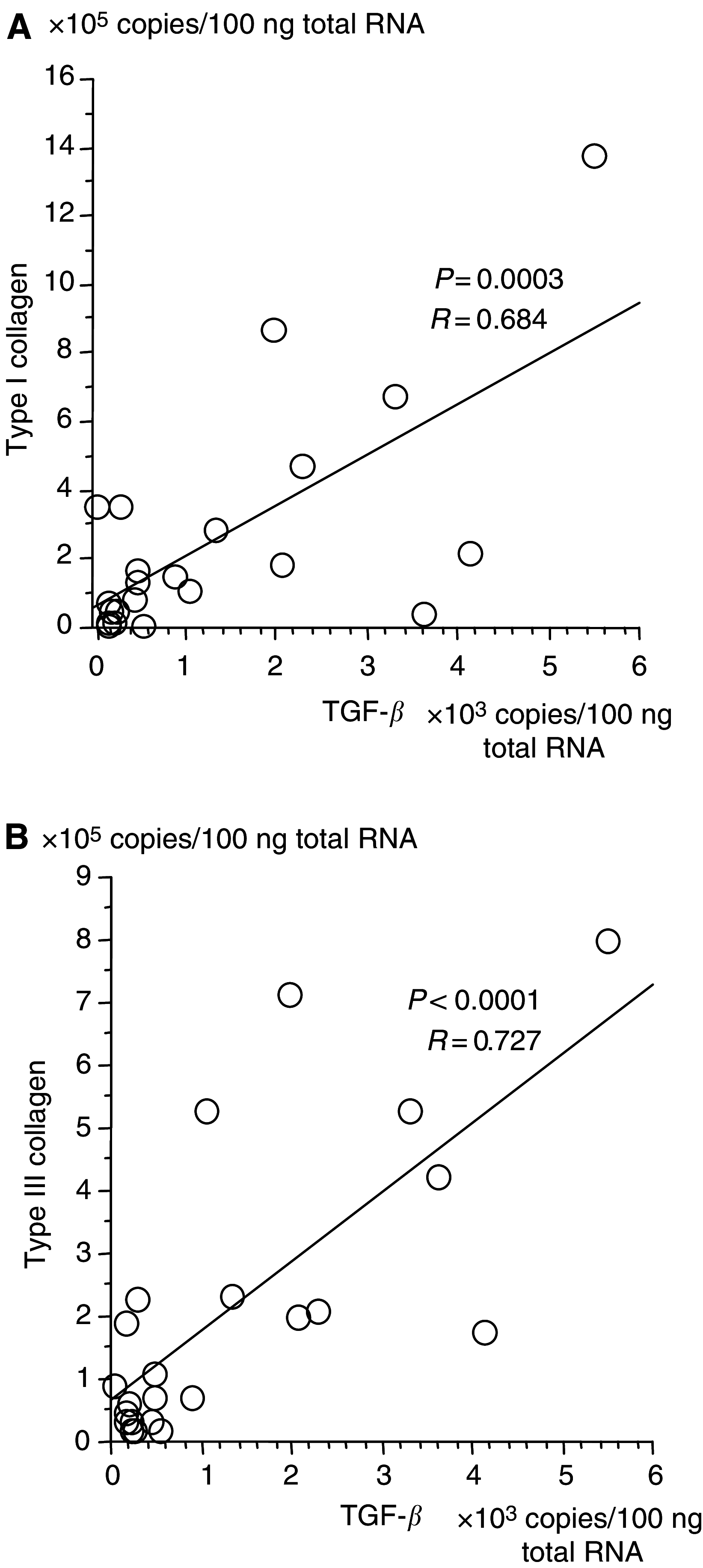
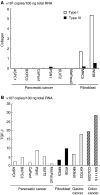
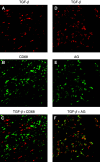
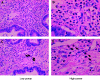
Pancreatic cancer is often associated with an intense production of interstitial collagens, known as the desmoplastic reaction. To understand more about desmoplasia in pancreatic cancer, the expression of mRNA for type I and III collagens and potent desmoplastic inducing growth factors transforming growth factor-beta (TGF-beta), connective tissue growth factor (CTGF), acidic and basic fibroblast growth factor (FGF), platelet-derived growth factor (PDGF) A and C and epidermal growth factor (EGF) was analysed by quantitative RT-PCR. Expression of both collagens in 23 frozen primary pancreatic cancer nodules was significantly higher than that in 15 non-neoplastic pancreatic tissues. The expressions of mRNAs for TGF-beta, acidic FGF, basic FGF and PDGF C were likewise higher in surgical cancer nodules, while that of CTGF, PDGF A and EGF were not. Among these growth factors, the expression of TGF-beta mRNA showed the most significant correlation with that of collagens (P<0.0001). By immunohistochemistry, TGF-beta showed faint cytoplasmic staining in cancer cells. In contrast, isolated cells, mainly located on the invasive front surrounding cancerous nests, were prominently and strongly stained. These TGF-beta-positive cells contained a segmented nucleus, were negative for anti-macrophage (CD68) and positive for anti-granulocyte antibodies, indicating their granulocytic nature. In conclusion, TGF-beta seemed to play a major role among the various growth factors in characteristic overproduction of collagens in pancreatic cancer. Moreover, the predominant cells that express TGF-beta were likely to be infiltrated granulocytes (mostly are neutrophils) and not pancreatic cancer cells.
Figures




References
-
- Appleton I, Tomlinson A, Colville-Nash PR, Willoughby DA (1993) Temporal and spatial immunolocalisation of cytokines in murine chronic granulomatous tissue. Implications for their role in tissue development and repair processes. Lab Invest 69: 405–414 - PubMed
-
- Coppola D, Lu L, Fruehauf JP, Kyshtoobayeva A, Karl RC, Nicosia SV, Yeatman TJ (1998) Analysis of p53, p21WAF1, and TGF-beta1 in human ductal adenocarcinoma of the pancreas: TGF-beta1 protein expression predicts longer survival. Am J Clin Pathol 110: 16–23 - PubMed
-
- Ebert M, Yokoyama M, Friess H, Kobrin MS, Buchler MW, Korc M (1995) Induction of platelet-derived growth factor A and B chains and over-expression of their receptors in human pancreatic cancer. Int J Cancer 62: 529–535 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

