High dose chemotherapy and autologous stem cell transplantation for poor risk and recurrent non-Hodgkin's lymphoma: a single-center experience of 50 patients
- PMID: 15366643
- PMCID: PMC4531588
- DOI: 10.3904/kjim.2004.19.2.114
High dose chemotherapy and autologous stem cell transplantation for poor risk and recurrent non-Hodgkin's lymphoma: a single-center experience of 50 patients
Abstract
Background: The long-term survival of patients with non-Hodgkin's lymphoma after conventional chemotherapy is about 35%, with the remaining 65% of patients tending to be refractory or experience relapse. As such, primary refractory patients responding to salvage chemotherapy, and sensitive relapsed patients and primary high-risk patients are recommended to receive high-dose chemotherapy (HDC) and autologous peripheral blood stem cell transplantation (PBSCT). We evaluated the role of HDC and autologous PBSCT in patients with primary refractory, primary high risk, and sensitive relapsed non-Hodgkin's lymphoma.
Methods: We performed a retrospective analysis of the data from 50 patients with non-Hodgkin's lymphoma who were treated with HDC and autologous PBSCT in the Catholic Hematopoietic Stem Cell Transplantation Center between 1997 and 2002.
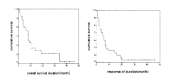
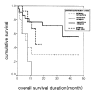
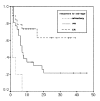
Results: Of the 50 patients, the conditioning regimen was BEAM in 20, CMT (cyclophosphamide, melphalan and thiotepa) in 19, fludarabine- and total body irradiation (TBI)-based regimen in 8, and cyclophosphamide and TBI in 2. There were 3 (6%) deaths due to treatment-related toxicity within the first 50 days after transplantation. Twenty-five patients remain alive at a median follow-up duration of 40.5 months (range 9-61). Among the patients with partial response before transplantation, 76% showed further response after transplantation. In half of these responders, the disease state was changed into complete response (CR) after transplantation. 2-year overall survival was 52% and 2-year progression free survival was 36.8%. Median overall survival was 34 months (range 8-60), and median progression-free survival was 8 months (range 1-14). Median overall survival was 14 months (range 9-19) in the primary high-risk group (n=13), 7 months (range 4-10) in the resistance relapse group (n=5), and 6 months (range 0-14) in the primary refractory group (n=10). Overall survival in the sensitive relapse group (n=22) did not reach the median; the mean overall survival in this group was 33 months. The disease status before transplantation was the only significant prognostic factor in determining overall survival (p=0.032) and progression- free survival (p=0.001).
Conclusion: HDC and autologous PBSCT appears to produce high response rate. Primary high-risk group and sensitive relapse group had good prognosis, while refractory and resistance relapse group had poor prognosis. And the pre-transplantation disease status was the only significant prognostic factor in multivariate analysis.
Figures
References
-
- Vose JM, Armitage JO. The present status of therapy for patients with aggressive non-Hodgkin’s lymphoma. Ann Oncol. 1991;2:171–176. - PubMed
-
- Fisher RI, Gaynor ER, Dahlberg S, Oken MM, Grogan TM, Mize EM, Glick JH, Coltman CA, Jr, Miller TP. Comparison of a standard regimen (CHOP) with three intensive chemotherapy regimens for advanced non-Hodgkin’s disease. N Engl J Med. 1993;328:1002–1006. - PubMed
-
- Longo DL, DeVita VT, Jr, Duffey PL, Wesley MN, Ihde DC, Hubbard SM, Gilliom M, Jaffe ES, Cossman J, Fisher RI. Superior of proMACE-cytoBOM over ProMACE-MOPP in the treatment of advanced diffuse aggressive lymphoma: results of a prospective randomized trial. J Clin Oncol. 1991;9:25–38. - PubMed
-
- Cabanillas F, Hagemeister FB, Bodey G, Freireich EJ. IMVP-16: an effective regimen for patients with lymphoma who have relapsed after initial combination chemotherapy. Blood. 1982;60:693–697. - PubMed
-
- Cabanillas F, Hagemeister FB, McLaughlin P, Velasquez WS, Riggs S, Fuller L, Smith T. Results of MINE salvage regimen for recurrent or refractory lymphoma. J Clin Oncol. 1987;5:407–412. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
