Human T-cell leukemia virus type 1 (HTLV-1) and HTLV-2 tax oncoproteins modulate cell cycle progression and apoptosis
- PMID: 15367606
- PMCID: PMC516438
- DOI: 10.1128/JVI.78.19.10399-10409.2004
Human T-cell leukemia virus type 1 (HTLV-1) and HTLV-2 tax oncoproteins modulate cell cycle progression and apoptosis
Abstract
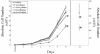
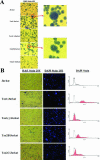
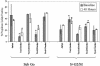
Human T-cell leukemia virus type 1 (HTLV-1) is the etiologic agent of adult T-cell leukemia and lymphoma, an aggressive clonal malignancy of human CD4-bearing T lymphocytes. HTLV-2, although highly related to HTLV-1 at the molecular level, has not been conclusively linked to development of lymphoproliferative disorders. Differences between the biological activities of the respective tax gene products (Tax1 and Tax2) may be one factor which accounts for the differential pathogenicities associated with infection. To develop an in vitro model to investigate and compare the effects of constitutive expression of Tax1 and Tax2, Jurkat T-cell lines were infected with lentivirus vectors encoding Tax1 and Tax2 in conjunction with green fluorescent protein, and stably transduced clonal cell lines were generated by serial dilution in the absence of drug selection. Jurkat cells that constitutively express Tax1 and Tax2 (Tax1/Jurkat and Tax2/Jurkat, respectively) showed notably reduced kinetics of cellular replication, and Tax1 inhibited cellular replication to a higher degree in comparison to Tax2. Tax1 markedly activated transcription from the cdk inhibitor p21(cip1/waf1) promoter in comparison to Tax2, suggesting that upregulation of p21(cip1/waf1) may account for the differential inhibition of cellular replication kinetics displayed by Tax1/Jurkat and Tax2/Jurkat cells. The presence of binucleated and multinucleated cells, reminiscent of large lymphocytes with cleaved or cerebriform nuclei often seen in HTLV-1- and -2-seropositive patients, was noted in cultures expressing Tax1 and Tax2. Although Tax1 and Tax2 expression mediated elevated resistance to apoptosis in Jurkat cells after serum deprivation, Tax1 was unique in protection from apoptosis after exposure to camptothecin and etoposide, inhibitors of topoisomerase I and II, respectively. Characterization of the unique phenotypes displayed by Tax1 and Tax2 in vitro will provide information as to the relative roles of these oncoproteins and their contribution to HTLV-1 and -2 pathogenesis in vivo.
Figures



Similar articles
-
Induction of cell cycle arrest by human T-cell lymphotropic virus type 1 Tax in hematopoietic progenitor (CD34+) cells: modulation of p21cip1/waf1 and p27kip1 expression.J Virol. 2005 Nov;79(22):14069-78. doi: 10.1128/JVI.79.22.14069-14078.2005. J Virol. 2005. PMID: 16254341 Free PMC article.
-
Human T-cell leukemia virus type 1 tax oncoprotein suppression of multilineage hematopoiesis of CD34+ cells in vitro.J Virol. 2003 Nov;77(22):12152-64. doi: 10.1128/jvi.77.22.12152-12164.2003. J Virol. 2003. PMID: 14581552 Free PMC article.
-
Human T cell leukemia virus type 2 (HTLV-2) Tax2 has a dominant activity over HTLV-1 Tax1 to immortalize human CD4+ T cells.Virus Genes. 2013 Feb;46(1):39-46. doi: 10.1007/s11262-012-0831-9. Epub 2012 Sep 29. Virus Genes. 2013. PMID: 23054433
-
Distinct functions of HTLV-1 Tax1 from HTLV-2 Tax2 contribute key roles to viral pathogenesis.Retrovirology. 2009 Dec 17;6:117. doi: 10.1186/1742-4690-6-117. Retrovirology. 2009. PMID: 20017952 Free PMC article. Review.
-
HTLV-1 and associated adult T-cell leukemia/lymphoma.Rev Clin Exp Hematol. 2003 Dec;7(4):336-61. Rev Clin Exp Hematol. 2003. PMID: 15129647 Review.
Cited by
-
Kinase Substrate Profiling Using a Proteome-wide Serine-Oriented Human Peptide Library.Biochemistry. 2018 Aug 7;57(31):4717-4725. doi: 10.1021/acs.biochem.8b00410. Epub 2018 Jun 19. Biochemistry. 2018. PMID: 29920078 Free PMC article.
-
Cell Immortality: In Vitro Effective Techniques to Achieve and Investigate Its Applications and Challenges.Life (Basel). 2024 Mar 21;14(3):417. doi: 10.3390/life14030417. Life (Basel). 2024. PMID: 38541741 Free PMC article. Review.
-
Human T-cell leukemia virus type 1 (HTLV-1) p12I down-modulates ICAM-1 and -2 and reduces adherence of natural killer cells, thereby protecting HTLV-1-infected primary CD4+ T cells from autologous natural killer cell-mediated cytotoxicity despite the reduction of major histocompatibility complex class I molecules on infected cells.J Virol. 2007 Sep;81(18):9707-17. doi: 10.1128/JVI.00887-07. Epub 2007 Jul 3. J Virol. 2007. PMID: 17609265 Free PMC article. Clinical Trial.
-
Tax gene expression and cell cycling but not cell death are selected during HTLV-1 infection in vivo.Retrovirology. 2010 Mar 11;7:17. doi: 10.1186/1742-4690-7-17. Retrovirology. 2010. PMID: 20222966 Free PMC article.
-
Induction of cell cycle arrest by human T-cell lymphotropic virus type 1 Tax in hematopoietic progenitor (CD34+) cells: modulation of p21cip1/waf1 and p27kip1 expression.J Virol. 2005 Nov;79(22):14069-78. doi: 10.1128/JVI.79.22.14069-14078.2005. J Virol. 2005. PMID: 16254341 Free PMC article.
References
-
- Akagi, T., H. Ono, and K. Shimotohno. 1996. Expression of cell-cycle regulatory genes in HTLV-1-infected T-cell lines: possible involvement of Tax1 in the altered expression of cyclin D2, p18Ink4, and p21Waf1/Cip1/Sdi1. Oncogene 12:1645-1652. - PubMed
-
- Ariumi, Y., A. Kaida, J. Y. Lin, M. Hirota, O. Masui, S. Yamaoka, Y. Taya, and K. Shimotohno. 2000. HTLV-1 tax oncoprotein represses the p53-mediated trans-activation function through coactivator CBP sequestration. Oncogene 19:1491-1499. - PubMed
-
- Buchschacher, G. L., Jr., and F. Wong-Staal. 2000. Development of lentiviral vectors for gene therapy for human diseases. Blood 95:2499-2504. - PubMed
-
- Burns, J. C., T. Friedmann, W. Driever, M. Burrascano, and J. K. Yee. 1993. Vesicular stomatitis virus G glycoprotein pseudotyped retroviral vectors: concentration to very high titer and efficient gene transfer into mammalian and nonmammalian cells. Proc. Natl. Acad. Sci. USA 90:8033-8037. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

