Murine coronavirus nonstructural protein p28 arrests cell cycle in G0/G1 phase
- PMID: 15367607
- PMCID: PMC516409
- DOI: 10.1128/JVI.78.19.10410-10419.2004
Murine coronavirus nonstructural protein p28 arrests cell cycle in G0/G1 phase
Abstract
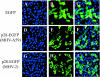
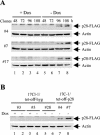
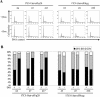
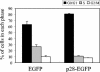
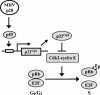
Murine coronavirus mouse hepatitis virus (MHV) gene 1 encodes several nonstructural proteins. The functions are unknown for most of these nonstructural proteins, including p28, which is encoded at the 5' end of the MHV genome. Transient expression of cloned p28 in several different cultured cells inhibited cell growth, indicating that p28 expression suppressed cell proliferation. Expressed p28 was exclusively localized in the cytoplasm. Cell cycle analysis by flow cytometry demonstrated that p28 expression induced G(0)/G(1) cell cycle arrest. Characterization of various cellular proteins that are involved in regulating cell cycle progression demonstrated that p28 expression resulted in an accumulation of hypophosphorylated retinoblastoma protein (pRb), tumor suppressor p53, and cyclin-dependent kinase (Cdk) inhibitor p21(Cip1). Expression of p28 did not alter the amount of p53 transcripts yet increased the amount of p21(Cip1) transcripts, suggesting that p28 expression increased p53 stability and that p21(Cip1) was transcriptionally activated in a p53-dependent manner. Our present data suggest the following model of p28-induced G(0)/G(1) cell cycle arrest. Expressed cytoplasmic p28 induces the stabilization of p53, and accumulated p53 causes transcriptional upregulation of p21(Cip1). The increased amount of p21(Cip1) suppresses cyclin E/Cdk2 activity, resulting in the inhibition of pRb hyperphosphorylation. Accumulation of hypophosphorylated pRb thus prevents cell cycle progression from G(0)/G(1) to S phase.
Figures



References
-
- Albrecht, T., and T. H. Weller. 1980. Heterogeneous morphologic features of plaques induced by five strains of human cytomegalovirus. Am. J. Clin. Pathol. 73:648-654. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

