Low pH is required for avian sarcoma and leukosis virus Env-dependent viral penetration into the cytosol and not for viral uncoating
- PMID: 15367609
- PMCID: PMC516436
- DOI: 10.1128/JVI.78.19.10433-10441.2004
Low pH is required for avian sarcoma and leukosis virus Env-dependent viral penetration into the cytosol and not for viral uncoating
Abstract
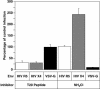
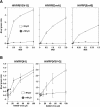
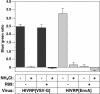
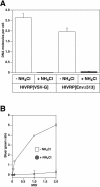
A novel entry mechanism has been proposed for the avian sarcoma and leukosis virus (ASLV), whereby interaction with specific cell surface receptors activates or primes the viral envelope glycoprotein (Env), rendering it sensitive to subsequent low-pH-dependent fusion triggering in acidic intracellular organelles. However, ASLV fusion seems to proceed to a lipid mixing stage at neutral pH, leading to the suggestion that low pH might instead be required for a later stage of viral entry such as uncoating (L. J. Earp, S. E. Delos, R. C. Netter, P. Bates, and J. M. White. J. Virol. 77:3058-3066, 2003). To address this possibility, hybrid virus particles were generated with the core of human immunodeficiency virus type 1 (HIV-1), a known pH-independent virus, and with subgroups A or B ASLV Env proteins. Infection of cells by these pseudotyped virions was blocked by lysosomotropic agents, as judged by inhibition of HIV-1 DNA synthesis. Furthermore, by using HIV-1 cores that contain a Vpr-beta-lactamase fusion protein (Vpr-BlaM) to monitor viral penetration into the cytosol, we demonstrated that virions bearing ASLV Env, but not HIV-1 Env, enter the cytosol in a low-pH-dependent manner. This effect was independent of the presence of the cytoplasmic tail of ASLV Env. These studies provide strong support for the model, indicating that low pH is required for ASLV Env-dependent viral penetration into the cytosol and not for viral uncoating.
Figures


References
-
- Barnard, R. J. O., and J. A. T. Young. 2003. Alpharetroviral receptor-envelope interactions. Curr. Top. Immunol. Microbiol. 281:107-136. - PubMed
-
- Brojatsch, J., J. Naughton, M. M. Rolls, K. Zingler, and J. A. T. Young. 1996. CAR1, a TNFR-related protein, is a cellular receptor for cytopathic avian leukosis-sarcoma viruses and mediates apoptosis. Cell 87:845-855. - PubMed
-
- Cavrios, M., C. de Noronha, and W. C. Greene. 2002. A sensitive and specific enzyme-based assay detecting HIV-1 virion fusion in primary T lymphocytes. Nat. Biotechnol. 20:1151-1154. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

