Induction of antiviral immunity by double-stranded RNA in a marine invertebrate
- PMID: 15367610
- PMCID: PMC516398
- DOI: 10.1128/JVI.78.19.10442-10448.2004
Induction of antiviral immunity by double-stranded RNA in a marine invertebrate
Abstract
Vertebrates mount a strong innate immune response against viruses, largely by activating the interferon system. Double-stranded RNA (dsRNA), a common intermediate formed during the life cycle of many viruses, is a potent trigger of this response. In contrast, no general inducible antiviral defense mechanism has been reported in any invertebrate. Here we show that dsRNA induces antiviral protection in the marine crustacean Litopenaeus vannamei. When treated with dsRNA, shrimp showed increased resistance to infection by two unrelated viruses, white spot syndrome virus and Taura syndrome virus. Induction of this antiviral state is independent of the sequence of the dsRNA used and therefore distinct from the sequence-specific dsRNA-mediated genetic interference phenomenon. This demonstrates for the first time that an invertebrate immune system, like its vertebrate counterparts, can recognize dsRNA as a virus-associated molecular pattern, resulting in the activation of an innate antiviral response.
Figures

 ; accession no. AJ312200), a 1,316-bp genomic noncoding region of clone BAC6 from the catfish IgH locus (•; accession no. CC936713), a 1,079-bp portion of pig IgG cDNA (✽; accession no. U03778), and a 1,184-bp fragment of the BAC cloning vector pBeloBAC11 (26) (♦). The chi-square statistic was used to assess the significance of the observed antiviral protection by comparing the final cumulative mortality in dsRNA-treated groups with that of their respective positive controls: (a) duck Igυ dsRNA (χ2 = 6.05 [0.01 < P < 0.025]) and duck Igυ dsRNA TSVx1000 (χ2 = 0.75 [not significant]) and (b) duck Igυ dsRNA (χ2 = 5.29 [0.01 < P < 0.025]), pig Ig dsRNA (χ2 = 12.93 [P < 0.001]), pBeloBAC11 dsRNA (χ2 = 8.67 [0.001 < P < 0.005]), and fish noncoding dsRNA (χ2 = 15.39 [P < 0.001]). Panel c shows eosin-hematoxylin staining and anti-WSSV immunostaining of hemopoietic tissue sections from a control infected shrimp in comparison to a dsRNA-treated/WSSV-infected shrimp. The sections shown were obtained 72 h after viral infection. Arrows indicate the presence of intranuclear inclusions characteristic of WSSV infection and WSSV-positive immunoreactivity. Scale bars, 20 μm.
; accession no. AJ312200), a 1,316-bp genomic noncoding region of clone BAC6 from the catfish IgH locus (•; accession no. CC936713), a 1,079-bp portion of pig IgG cDNA (✽; accession no. U03778), and a 1,184-bp fragment of the BAC cloning vector pBeloBAC11 (26) (♦). The chi-square statistic was used to assess the significance of the observed antiviral protection by comparing the final cumulative mortality in dsRNA-treated groups with that of their respective positive controls: (a) duck Igυ dsRNA (χ2 = 6.05 [0.01 < P < 0.025]) and duck Igυ dsRNA TSVx1000 (χ2 = 0.75 [not significant]) and (b) duck Igυ dsRNA (χ2 = 5.29 [0.01 < P < 0.025]), pig Ig dsRNA (χ2 = 12.93 [P < 0.001]), pBeloBAC11 dsRNA (χ2 = 8.67 [0.001 < P < 0.005]), and fish noncoding dsRNA (χ2 = 15.39 [P < 0.001]). Panel c shows eosin-hematoxylin staining and anti-WSSV immunostaining of hemopoietic tissue sections from a control infected shrimp in comparison to a dsRNA-treated/WSSV-infected shrimp. The sections shown were obtained 72 h after viral infection. Arrows indicate the presence of intranuclear inclusions characteristic of WSSV infection and WSSV-positive immunoreactivity. Scale bars, 20 μm.
 ; accession no. AJ312200), a 1,316-bp genomic noncoding region of clone BAC6 from the catfish IgH locus (•; accession no. CC936713), a 1,079-bp portion of pig IgG cDNA (✽; accession no. U03778), and a 1,184-bp fragment of the BAC cloning vector pBeloBAC11 (26) (♦). The chi-square statistic was used to assess the significance of the observed antiviral protection by comparing the final cumulative mortality in dsRNA-treated groups with that of their respective positive controls: (a) duck Igυ dsRNA (χ2 = 6.05 [0.01 < P < 0.025]) and duck Igυ dsRNA TSVx1000 (χ2 = 0.75 [not significant]) and (b) duck Igυ dsRNA (χ2 = 5.29 [0.01 < P < 0.025]), pig Ig dsRNA (χ2 = 12.93 [P < 0.001]), pBeloBAC11 dsRNA (χ2 = 8.67 [0.001 < P < 0.005]), and fish noncoding dsRNA (χ2 = 15.39 [P < 0.001]). Panel c shows eosin-hematoxylin staining and anti-WSSV immunostaining of hemopoietic tissue sections from a control infected shrimp in comparison to a dsRNA-treated/WSSV-infected shrimp. The sections shown were obtained 72 h after viral infection. Arrows indicate the presence of intranuclear inclusions characteristic of WSSV infection and WSSV-positive immunoreactivity. Scale bars, 20 μm.
; accession no. AJ312200), a 1,316-bp genomic noncoding region of clone BAC6 from the catfish IgH locus (•; accession no. CC936713), a 1,079-bp portion of pig IgG cDNA (✽; accession no. U03778), and a 1,184-bp fragment of the BAC cloning vector pBeloBAC11 (26) (♦). The chi-square statistic was used to assess the significance of the observed antiviral protection by comparing the final cumulative mortality in dsRNA-treated groups with that of their respective positive controls: (a) duck Igυ dsRNA (χ2 = 6.05 [0.01 < P < 0.025]) and duck Igυ dsRNA TSVx1000 (χ2 = 0.75 [not significant]) and (b) duck Igυ dsRNA (χ2 = 5.29 [0.01 < P < 0.025]), pig Ig dsRNA (χ2 = 12.93 [P < 0.001]), pBeloBAC11 dsRNA (χ2 = 8.67 [0.001 < P < 0.005]), and fish noncoding dsRNA (χ2 = 15.39 [P < 0.001]). Panel c shows eosin-hematoxylin staining and anti-WSSV immunostaining of hemopoietic tissue sections from a control infected shrimp in comparison to a dsRNA-treated/WSSV-infected shrimp. The sections shown were obtained 72 h after viral infection. Arrows indicate the presence of intranuclear inclusions characteristic of WSSV infection and WSSV-positive immunoreactivity. Scale bars, 20 μm.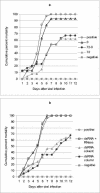

References
-
- Adams, M. D., S. E. Celniker, R. A. Holt, C. A. Evans, J. D. Gocayne, P. G. Amanatides, S. E. Scherer, P. W. Li, R. A. Hoskins, R. F. Galle, R. A. George, S. E. Lewis, S. Richards, M. Ashburner, S. N. Henderson, G. G. Sutton, J. R. Wortman, M. D. Yandell, Q. Zhang, L. X. Chen, R. C. Brandon, Y. H. Rogers, R. G. Blazej, M. Champe, B. D. Pfeiffer, K. H. Wan, C. Doyle, E. G. Baxter, G. Helt, C. R. Nelson, G. L. Gabor, J. F. Abril, A. Agbayani, H. J. An, C. Andrews-Pfannkoch, D. Baldwin, R. M. Ballew, A. Basu, J. Baxendale, L. Bayraktaroglu, E. M. Beasley, K. Y. Beeson, P. V. Benos, B. P. Berman, D. Bhandari, S. Bolshakov, D. Borkova, M. R. Botchan, J. Bouck, P. Brokstein, P. Brottier, K. C. Burtis, D. A. Busam, H. Butler, E. Cadieu, A. Center, I. Chandra, J. M. Cherry, S. Cawley, C. Dahlke, L. B. Davenport, P. Davies, B. de Pablos, A. Delcher, Z. Deng, A. D. Mays, I. Dew, S. M. Dietz, K. Dodson, L. E. Doup, M. Downes, S. Dugan-Rocha, B. C. Dunkov, P. Dunn, K. J. Durbin, C. C. Evangelista, C. Ferraz, S. Ferriera, W. Fleischmann, C. Fosler, A. E. Gabrielian, N. S. Garg, W. M. Gelbart, K. Glasser, A. Glodek, F. Gong, J. H. Gorrell, Z. Gu, P. Guan, M. Harris, N. L. Harris, D. Harvey, T. J. Heiman, J. R. Hernandez, J. Houck, D. Hostin, K. A. Houston, T. J. Howland, M. H. Wei, C. Ibegwam, et al. 2000. The genome sequence of Drosophila melanogaster. Science 287:2185-2195. - PubMed
-
- Agaisse, H., U. M. Petersen, M. Boutros, B. Mathey-Prevot, and N. Perrimon. 2003. Signaling role of hemocytes in Drosophila JAK/STAT-dependent response to septic injury. Dev. Cell 5:441-450. - PubMed
-
- Akira, S., and H. Hemmi. 2003. Recognition of pathogen-associated molecular patterns by TLR family. Immunol. Lett. 85:85-95. - PubMed
-
- Alexopoulou, L., A. C. Holt, R. Medzhitov, and R. A. Flavell. 2001. Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature 413:732-738. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

