Memory T-cell-mediated immune responses specific to an alternative core protein in hepatitis C virus infection
- PMID: 15367612
- PMCID: PMC516386
- DOI: 10.1128/JVI.78.19.10460-10469.2004
Memory T-cell-mediated immune responses specific to an alternative core protein in hepatitis C virus infection
Abstract
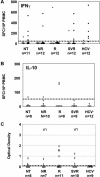
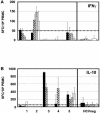
In vitro studies have described the synthesis of an alternative reading frame form of the hepatitis C virus (HCV) core protein that was named F protein or ARFP (alternative reading frame protein) and includes a domain coded by the +1 open reading frame of the RNA core coding region. The expression of this protein in HCV-infected patients remains controversial. We have analyzed peripheral blood from 47 chronically or previously HCV-infected patients for the presence of T lymphocytes and antibodies specific to the ARFP. Anti-ARFP antibodies were detected in 41.6% of the patients infected with various HCV genotypes. Using a specific ARFP 99-amino-acid polypeptide as well as four ARFP predicted class I-restricted 9-mer peptides, we show that 20% of the patients display specific lymphocytes capable of producing gamma interferon, interleukin-10, or both cytokines. Patients harboring three different viral genotypes (1a, 1b, and 3) carried T lymphocytes reactive to genotype 1b-derived peptides. In longitudinal analysis of patients receiving therapy, both core and ARFP-specific T-cell- and B-cell-mediated responses were documented. The magnitude and kinetics of the HCV antigen-specific responses differed and were not linked with viremia or therapy outcome. These observations provide strong and new arguments in favor of the synthesis, during natural HCV infection, of an ARFP derived from the core sequence. Moreover, the present data provide the first demonstration of the presence of T-cell-mediated immune responses directed to this novel HCV antigen.
Figures




Similar articles
-
Effects of interferon treatment on the antiviral T-cell response in hepatitis C virus genotype 1b- and genotype 2c-infected patients.Hepatology. 1997 Sep;26(3):792-7. doi: 10.1053/jhep.1997.v26.pm0009303515. Hepatology. 1997. PMID: 9303515
-
T- and B-cell responses to different hepatitis C virus antigens in patients with chronic hepatitis C infection and in healthy anti-hepatitis C virus--positive blood donors without viremia.Hepatology. 1996 Oct;24(4):790-5. doi: 10.1002/hep.510240406. Hepatology. 1996. PMID: 8855177
-
Assessment of specific antibodies to F protein in serum samples from Chinese hepatitis C patients treated with interferon plus ribavarin.J Clin Microbiol. 2008 Nov;46(11):3746-51. doi: 10.1128/JCM.00612-08. Epub 2008 Oct 1. J Clin Microbiol. 2008. PMID: 18832124 Free PMC article.
-
The hepatitis C virus alternate reading frame (ARF) and its family of novel products: the alternate reading frame protein/F-protein, the double-frameshift protein, and others.Semin Liver Dis. 2005 Feb;25(1):105-17. doi: 10.1055/s-2005-864786. Semin Liver Dis. 2005. PMID: 15732002 Review.
-
The HCV ARFP/F/core+1 protein: production and functional analysis of an unconventional viral product.IUBMB Life. 2009 Jul;61(7):739-52. doi: 10.1002/iub.201. IUBMB Life. 2009. PMID: 19548320 Review.
Cited by
-
Translation of the F protein of hepatitis C virus is initiated at a non-AUG codon in a +1 reading frame relative to the polyprotein.Nucleic Acids Res. 2005 Mar 8;33(5):1474-86. doi: 10.1093/nar/gki292. Print 2005. Nucleic Acids Res. 2005. PMID: 15755749 Free PMC article.
-
Alternative translational reading frames as a novel source of epitopes for an expanded CD8 T-cell repertoire: use of a retroviral system to assess the translational requirements for CTL recognition and lysis.Viral Immunol. 2010 Dec;23(6):577-83. doi: 10.1089/vim.2010.0057. Viral Immunol. 2010. PMID: 21142443 Free PMC article.
-
Relationship between antibodies to hepatitis C virus core+1 protein and treatment outcome.Ann Gastroenterol. 2018 Sep-Oct;31(5):593-597. doi: 10.20524/aog.2018.0290. Epub 2018 Jul 13. Ann Gastroenterol. 2018. PMID: 30174396 Free PMC article.
-
The major form of hepatitis C virus alternate reading frame protein is suppressed by core protein expression.Nucleic Acids Res. 2008 May;36(9):3054-64. doi: 10.1093/nar/gkn111. Epub 2008 Apr 8. Nucleic Acids Res. 2008. PMID: 18400784 Free PMC article.
-
Sero-reactivity to three distinct regions within the hepatitis C virus alternative reading frame protein (ARFP/core+1) in patients with chronic HCV genotype-3 infection.J Gen Virol. 2022 Mar;103(3):001727. doi: 10.1099/jgv.0.001727. J Gen Virol. 2022. PMID: 35230930 Free PMC article.
References
-
- Bain, C., A. Fatmi, F. Zoulim, J. P. Zarski, C. Trépo, and G. Inchauspé. 2001. Impaired allostimulatory function of dendritic cells in chronic hepatitis C infection. Gastroenterology 120:512-524. - PubMed
-
- Barnes, E., G. Harcourt, D. Brown, M. Lucas, R. Phillips, G. Dusheiko, and P. Klenerman. 2002. The dynamics of T-lymphocyte responses during combination therapy for chronic hepatitis C virus infection. Hepatology 36:743-754. - PubMed
-
- Boaz, M. J., A. Waters, S. Murad, P. J. Easterbrook, and A. Vyakarnam. 2002. Presence of HIV-1 Gag-specific IFN-gamma+IL-2+ and CD28+IL-2+ CD4 T cell responses is associated with nonprogression in HIV-1 infection. J. Immunol. 169:6376-6385. - PubMed
-
- Boulant, S., M. Becchi, F. Penin, and J. Lavergne. 2003. Unusual multiple recoding events leading to alternative forms of hepatitis C virus core protein from genotype 1b. J. Biol. Chem. 278:45785-45792. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

