Dual role of prostratin in inhibition of infection and reactivation of human immunodeficiency virus from latency in primary blood lymphocytes and lymphoid tissue
- PMID: 15367617
- PMCID: PMC516376
- DOI: 10.1128/JVI.78.19.10507-10515.2004
Dual role of prostratin in inhibition of infection and reactivation of human immunodeficiency virus from latency in primary blood lymphocytes and lymphoid tissue
Abstract
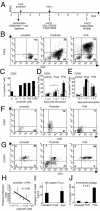
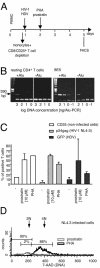
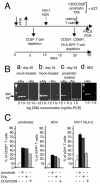
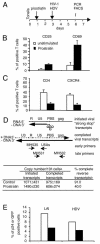
To design strategies to purge latent reservoirs of human immunodeficiency virus type 1 (HIV-1), we investigated mechanisms by which a non-tumor-promoting phorbol ester, prostratin, inhibits infection of CD4(+) T lymphocytes and at the same time reactivates virus from latency. CD4(+) T lymphocytes from primary blood mononuclear cells (PBMC) and in blocks of human lymphoid tissue were stimulated with prostratin and infected with HIV-1 to investigate the effects of prostratin on cellular susceptibility to the virus. The capacity of prostratin to reactivate HIV from latency was tested in CD4(+) T cells harboring preintegrated and integrated latent provirus. Prostratin stimulated CD4(+) T cells in an aberrant way. It induced expression of the activation markers CD25 and CD69 but inhibited cell cycling. HIV-1 uptake was reduced in prostratin-stimulated CD4(+) T PBMC and tissues in a manner consistent with a downregulation of CD4 and CXCR4 receptors in these systems. At the postentry level, prostratin inhibited completion of reverse transcription of the viral genome in lymphoid tissue. However, prostratin facilitated integration of the reverse-transcribed HIV-1 genome in nondividing CD4(+) T cells and facilitated expression of already integrated HIV-1, including latent forms. Thus, while stimulation with prostratin restricts susceptibility of primary resting CD4(+) T cells to HIV infection at the virus cell-entry level and at the reverse transcription level, it efficiently reactivates HIV-1 from pre- and postintegration latency in resting CD4(+) T cells.
Figures
Similar articles
-
Differential effects of phorbol-13-monoesters on human immunodeficiency virus reactivation.Biochem Pharmacol. 2008 Mar 15;75(6):1370-80. doi: 10.1016/j.bcp.2007.12.004. Epub 2007 Dec 23. Biochem Pharmacol. 2008. PMID: 18241838
-
Effects of prostratin on T-cell activation and human immunodeficiency virus latency.J Virol. 2002 Aug;76(16):8118-23. doi: 10.1128/jvi.76.16.8118-8123.2002. J Virol. 2002. PMID: 12134017 Free PMC article.
-
Gradual shutdown of virus production resulting in latency is the norm during the chronic phase of human immunodeficiency virus replication and differential rates and mechanisms of shutdown are determined by viral sequences.Virology. 1996 Nov 1;225(1):196-212. doi: 10.1006/viro.1996.0588. Virology. 1996. PMID: 8918547
-
Prostratin as a new therapeutic agent targeting HIV viral reservoirs.Drug News Perspect. 2005 Oct;18(8):496-500. doi: 10.1358/dnp.2005.18.8.944543. Drug News Perspect. 2005. PMID: 16391719 Review.
-
Experimental approaches to the study of HIV-1 latency.Nat Rev Microbiol. 2007 Feb;5(2):95-106. doi: 10.1038/nrmicro1580. Nat Rev Microbiol. 2007. PMID: 17224919 Review.
Cited by
-
Chemical Constituents of Root Barks of Gnidia involucrata and Evaluation for Antibacterial and Antioxidant Activities.J Trop Med. 2019 Aug 14;2019:8486214. doi: 10.1155/2019/8486214. eCollection 2019. J Trop Med. 2019. PMID: 31485237 Free PMC article.
-
Combinatorial latency reactivation for HIV-1 subtypes and variants.J Virol. 2010 Jun;84(12):5958-74. doi: 10.1128/JVI.00161-10. Epub 2010 Mar 31. J Virol. 2010. PMID: 20357084 Free PMC article.
-
Synergistic activation of HIV-1 expression by deacetylase inhibitors and prostratin: implications for treatment of latent infection.PLoS One. 2009 Jun 30;4(6):e6093. doi: 10.1371/journal.pone.0006093. PLoS One. 2009. PMID: 19564922 Free PMC article.
-
Variability in content of the anti-AIDS drug candidate prostratin in Samoan populations of Homalanthus nutans.J Nat Prod. 2008 Dec;71(12):2041-4. doi: 10.1021/np800295m. J Nat Prod. 2008. PMID: 19007283 Free PMC article.
-
Targeting HIV latency: pharmacologic strategies toward eradication.Drug Discov Today. 2013 Jun;18(11-12):541-51. doi: 10.1016/j.drudis.2012.12.008. Epub 2012 Dec 25. Drug Discov Today. 2013. PMID: 23270785 Free PMC article. Review.
References
-
- Brooks, D. G., D. H. Hamer, P. A. Arlen, L. Gao, G. Bristol, C. M. Kitchen, E. A. Berger, and J. A. Zack. 2003. Molecular characterization, reactivation, and depletion of latent HIV. Immunity 19:413-423. - PubMed
-
- Butler, S. L., M. S. Hansen, and F. D. Bushman. 2001. A quantitative assay for HIV DNA integration in vivo. Nat. Med. 7:631-634. - PubMed
-
- Chun, T. W., L. Carruth, D. Finzi, X. Shen, J. A. DiGiuseppe, H. Taylor, M. Hermankova, K. Chadwick, J. Margolick, T. C. Quinn, Y. H. Kuo, R. Brookmeyer, M. A. Zeiger, P. Barditch-Crovo, and R. F. Siliciano. 1997. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature 387:183-188. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

