The raft-promoting property of virion-associated cholesterol, but not the presence of virion-associated Brij 98 rafts, is a determinant of human immunodeficiency virus type 1 infectivity
- PMID: 15367622
- PMCID: PMC516414
- DOI: 10.1128/JVI.78.19.10556-10565.2004
The raft-promoting property of virion-associated cholesterol, but not the presence of virion-associated Brij 98 rafts, is a determinant of human immunodeficiency virus type 1 infectivity
Abstract
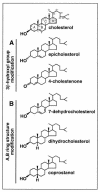
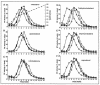
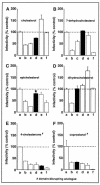
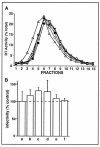
Lipid rafts are enriched in cholesterol and sphingomyelin and are isolated on the basis of insolubility in detergents, such as Brij 98 and Triton X-100. Recent work by Holm et al. has shown that rafts insoluble in Brig 98 can be found in human immunodeficiency virus type 1 (HIV-1) virus-like particles, although it is not known whether raft-like structures are present in authentic HIV-1 and it is unclear whether a virion-associated raft-like structure is required for HIV replication. Independently, it was previously reported that virion-associated cholesterol is critical for HIV-1 infectivity, although the specific requirement of virion cholesterol in HIV-1 was not examined. In the present study, we have demonstrated that infectious wild-type HIV-1 contains Brij 98 rafts but only minimal amounts of Triton X-100 rafts. To directly assess the functional requirement of virion-associated rafts and various features of cholesterol on HIV-1 replication, we replaced virion cholesterol with exogenous cholesterol analogues that have demonstrated either raft-promoting or -inhibiting capacity in model membranes. We observed that variable concentrations of exogenous analogues are required to replace a defined amount of virion-associated cholesterol, showing that structurally diverse cholesterol analogues have various affinities toward HIV-1. We found that replacement of 50% of virion cholesterol with these exogenous cholesterol analogues did not eliminate the presence of Brij 98 rafts in HIV-1. However, the infectivity levels of the lipid-modified HIV-1s directly correlate with the raft-promoting capacities of these cholesterol analogues. Our data provide the first direct assessment of virion-associated Brij 98 rafts in retroviral replication and illustrate the importance of the raft-promoting property of virion-associated cholesterol in HIV-1 replication.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

