Ring finger protein ZIN interacts with human immunodeficiency virus type 1 Vif
- PMID: 15367624
- PMCID: PMC516435
- DOI: 10.1128/JVI.78.19.10574-10581.2004
Ring finger protein ZIN interacts with human immunodeficiency virus type 1 Vif
Abstract
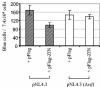
Virion infectivity factor (Vif) protein of human immunodeficiency virus type 1 (HIV-1) is essential for the productive infection of primary human CD4 T lymphocytes and macrophages. Vif overcomes the HIV-inhibitory effects of cellular factor APOBEC3G, which has cytidine deaminase activity. We previously reported the isolation of a Vif-interacting ring finger protein, Triad 3, from a human leukocyte cDNA library, using the yeast two-hybrid system. The full-length cellular protein homologue of Triad 3 has been recently identified as the zinc finger protein inhibiting NF-kappaB (ZIN). Sequence analysis indicates that Triad 3 protein contains all four major ring-like motifs of ZIN. We report here that ZIN binds to purified Vif in vitro and that Triad 3/ZIN interacts with HIV-1 Vif in transfected human 293T cells, as demonstrated by coimmunoprecipitation. To test the biological relevance of this interaction, we produced infectious HIV-1 NL4.3 in the presence or absence of cotransfected ZIN. HIV-1 NL4.3 virus stocks produced in the presence of exogenously expressed ZIN were twofold less infectious in a single-cycle infectivity assay than virus produced in the absence of exogenous ZIN. It was further shown that cells infected with HIV NL4.3 virus stocks produced in the presence of exogenously expressed ZIN were impaired in viral DNA synthesis by twofold. The impairment in viral reverse transcription and the reduction in single-cycle viral infectivity were both shown to be dependent on the presence of Vif in the virus producer cells. The possible mechanisms by which ZIN interferes with the early events of HIV-1 replication are discussed.
Figures



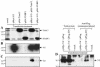

Similar articles
-
Isolation of a human gene that inhibits HIV-1 infection and is suppressed by the viral Vif protein.Nature. 2002 Aug 8;418(6898):646-50. doi: 10.1038/nature00939. Epub 2002 Jul 14. Nature. 2002. PMID: 12167863
-
A single amino acid substitution in human APOBEC3G antiretroviral enzyme confers resistance to HIV-1 virion infectivity factor-induced depletion.Proc Natl Acad Sci U S A. 2004 Apr 13;101(15):5652-7. doi: 10.1073/pnas.0400830101. Epub 2004 Mar 30. Proc Natl Acad Sci U S A. 2004. PMID: 15054139 Free PMC article.
-
Inhibition of tRNA₃(Lys)-primed reverse transcription by human APOBEC3G during human immunodeficiency virus type 1 replication.J Virol. 2006 Dec;80(23):11710-22. doi: 10.1128/JVI.01038-06. Epub 2006 Sep 13. J Virol. 2006. PMID: 16971427 Free PMC article.
-
The role of Vif during HIV-1 infection: interaction with novel host cellular factors.J Clin Virol. 2003 Feb;26(2):143-52. doi: 10.1016/s1386-6532(02)00113-0. J Clin Virol. 2003. PMID: 12600646 Review.
-
New insights into the role of Vif in HIV-1 replication.AIDS Rev. 2004 Jan-Mar;6(1):34-9. AIDS Rev. 2004. PMID: 15168739 Review.
Cited by
-
RNF157 attenuates CD4+ T cell-mediated autoimmune response by promoting HDAC1 ubiquitination and degradation.Theranostics. 2023 Jun 19;13(11):3509-3523. doi: 10.7150/thno.86307. eCollection 2023. Theranostics. 2023. PMID: 37441600 Free PMC article.
-
The dimerization domain of HIV-1 viral infectivity factor Vif is required to block virion incorporation of APOBEC3G.Retrovirology. 2007 Nov 24;4:81. doi: 10.1186/1742-4690-4-81. Retrovirology. 2007. PMID: 18036235 Free PMC article.
-
APOBEC3G multimers are recruited to the plasma membrane for packaging into human immunodeficiency virus type 1 virus-like particles in an RNA-dependent process requiring the NC basic linker.J Virol. 2007 May;81(10):5000-13. doi: 10.1128/JVI.02237-06. Epub 2007 Mar 7. J Virol. 2007. PMID: 17344295 Free PMC article.
-
Regulation of Apobec3F and human immunodeficiency virus type 1 Vif by Vif-Cul5-ElonB/C E3 ubiquitin ligase.J Virol. 2005 Aug;79(15):9579-87. doi: 10.1128/JVI.79.15.9579-9587.2005. J Virol. 2005. PMID: 16014920 Free PMC article.
-
The ring between ring fingers (RBR) protein family.Genome Biol. 2007;8(3):209. doi: 10.1186/gb-2007-8-3-209. Genome Biol. 2007. PMID: 17367545 Free PMC article. Review.
References
-
- Akari, H., M. Fujita, S. Kao, M. A. Khan, M. Shehu-Xhilaga, A. Adachi, and K. Strebel. 2004. High level expression of human immunodeficiency virus type-1 Vif inhibits viral infectivity by modulating proteolytic processing of the Gag precursor at the p2/nucleocapsid processing site. J. Biol. Chem. 279:12355-12362. - PubMed
-
- Blanc, D., C. Patience, T. F. Schulz, R. Weiss, and B. Spire. 1993. Transcomplementation of VIF- HIV-1 mutants in CEM cells suggests that VIF affects late steps of the viral life cycle. Virology 193:186-192. - PubMed
-
- Carr, J. M., H. Hocking, P. Li, and C. J. Burrell. 1999. Rapid and efficient cell-to-cell transmission of human immunodeficiency virus infection from monocyte-derived macrophages to peripheral blood lymphocytes. Virology 265:319-329. - PubMed
-
- Chen, D., X. Li, Z. Zhai, and H. B. Shu. 2002. A novel zinc finger protein interacts with receptor-interacting protein (RIP) and inhibits tumor necrosis factor (TNF)- and IL1-induced NF-kappa B activation. J. Biol. Chem. 277:15985-15991. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

