Profile of resistance of human immunodeficiency virus to mannose-specific plant lectins
- PMID: 15367629
- PMCID: PMC516383
- DOI: 10.1128/JVI.78.19.10617-10627.2004
Profile of resistance of human immunodeficiency virus to mannose-specific plant lectins
Erratum in
- J Virol.2004 Nov;78(2):12724. Böhlmstedt, Anders [corrected to Bölmstedt, Anders]
Abstract
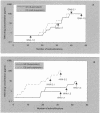
The mannose-specific plant lectins from the Amaryllidaceae family (e.g., Hippeastrum sp. hybrid and Galanthus nivalis) inhibit human immunodeficiency virus (HIV) infection of human lymphocytic cells in the higher nanogram per milliliter range and suppress syncytium formation between persistently HIV type 1 (HIV-1)-infected cells and uninfected CD4(+) T cells. These lectins inhibit virus entry. When exposed to escalating concentrations of G. nivalis and Hippeastrum sp. hybrid agglutinin, a variety of HIV-1(III(B)) strains were isolated after 20 to 40 subcultivations which showed a decreased sensitivity to the plant lectins. Several amino acid changes in the envelope glycoprotein gp120, but not in gp41, of the mutant virus isolates were observed. The vast majority of the amino acid changes occurred at the N glycosylation sites and at the S or T residues that are part of the N glycosylation motif. The degree of resistance to the plant lectins was invariably correlated with an increasing number of mutated glycosylation sites in gp120. The nature of these mutations was entirely different from that of mutations that are known to appear in HIV-1 gp120 under the pressure of other viral entry inhibitors such as dextran sulfate, bicyclams (i.e., AMD3100), and chicoric acid, which also explains the lack of cross-resistance of plant lectin-resistant viruses to any other HIV inhibitor including T-20 and the blue-green algae (cyanobacteria)-derived mannose-specific cyanovirin. The plant lectins represent a well-defined class of anti-HIV (microbicidal) drugs with a novel HIV drug resistance profile different from those of other existing anti-HIV drugs.
Figures
References
-
- Arendrup, M., A. Sönnerborg, B. Svennerholm, L. Åkerblom, C. Nielsen, H. Clausen, S. Olofsson, J. O. Nielsen, and J.-E. S. Hansen. 1993. Neutralizing antibody response during human immunodeficiency virus type 1 infection: type and group specificity and viral escape. J. Gen. Virol. 74:855-863. - PubMed
-
- Arthos, J., C. Cicala, T. D. Steenbeke, T. W. Chun, C. Dela Cruz, D. B. Hanback, P. Khazanie, D. Nam, P. Schuck, S. M. Selig, D. Van Ryk, M. A. Chaikin, and A. S. Fauci. 2002. Biochemical and biological characterization of a dodecameric CD4-Ig fusion protein: implications for therapeutic and vaccine strategies. J. Biol. Chem. 277:11456-11464. - PubMed
-
- Baba, M., O. Nishimura, N. Kanzaki, M. Okamoto, H. Sawada, Y. Iizawa, M. Shiraishi, Y. Aramaki, K. Okonogi, Y. Ogawa, K. Meguro, and M. Fujino. 1999. A small-molecule, nonpeptide CCR5 antagonist with highly potent and selective anti-HIV-1 activity. Proc. Natl. Acad. Sci. USA 96:5698-5703. - PMC - PubMed
-
- Balzarini, J., J. Neyts, D. Schols, M. Hosoya, E. Van Damme, W. Peumans, and E. De Clercq. 1992. The mannose-specific plant lectins from Cymbidium hybrid and Epipactis helleborine and the (N-acetylglucosamine)n-specific plant lectin from Urtica dioica are potent and selective inhibitors of human immunodeficiency virus and cytomegalovirus replication in vitro. Antivir. Res. 18:191-207. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

