The signal peptide of the Junín arenavirus envelope glycoprotein is myristoylated and forms an essential subunit of the mature G1-G2 complex
- PMID: 15367645
- PMCID: PMC516395
- DOI: 10.1128/JVI.78.19.10783-10792.2004
The signal peptide of the Junín arenavirus envelope glycoprotein is myristoylated and forms an essential subunit of the mature G1-G2 complex
Abstract
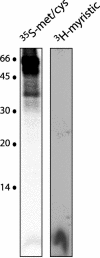
Arenaviruses comprise a diverse family of rodent-borne viruses that are responsible for recurring and emerging outbreaks of viral hemorrhagic fevers worldwide. The Junín virus, a member of the New World arenaviruses, is endemic to the pampas grasslands of Argentina and is the etiologic agent of Argentine hemorrhagic fever. In this study, we have analyzed the assembly and function of the Junín virus envelope glycoproteins. The mature envelope glycoprotein complex is proteolytically processed from the GP-C precursor polypeptide and consists of three noncovalently associated subunits, G1, G2, and a stable 58-amino-acid signal peptide. This tripartite organization is found both on virions of the attenuated Candid 1 strain and in cells expressing the pathogenic MC2 strain GP-C gene. Replacement of the Junín virus GP-C signal peptide with that of human CD4 has little effect on glycoprotein assembly while abolishing the ability of the G1-G2 complex to mediate pH-dependent cell-cell fusion. In addition, we demonstrate that the Junín virus GP-C signal peptide subunit is myristoylated at its N-terminal glycine. Alanine substitution for the modified glycine residue in the GP-C signal peptide does not affect formation of the tripartite envelope glycoprotein complex but markedly reduces its membrane fusion activity. In contrast to the classical view that signal peptides act primarily in targeting nascent polypeptides to the endoplasmic reticulum, we suggest that the signal peptide of the arenavirus GP-C may serve additional functions in envelope glycoprotein structure and trafficking.
Figures

Similar articles
-
Role of the stable signal peptide of Junín arenavirus envelope glycoprotein in pH-dependent membrane fusion.J Virol. 2006 Aug;80(15):7775-80. doi: 10.1128/JVI.00642-06. J Virol. 2006. PMID: 16840359 Free PMC article.
-
Role of the stable signal peptide and cytoplasmic domain of G2 in regulating intracellular transport of the Junín virus envelope glycoprotein complex.J Virol. 2006 Jun;80(11):5189-98. doi: 10.1128/JVI.00208-06. J Virol. 2006. PMID: 16698999 Free PMC article.
-
A novel zinc-binding domain is essential for formation of the functional Junín virus envelope glycoprotein complex.J Virol. 2007 Dec;81(24):13385-91. doi: 10.1128/JVI.01785-07. Epub 2007 Oct 10. J Virol. 2007. PMID: 17928348 Free PMC article.
-
The curious case of arenavirus entry, and its inhibition.Viruses. 2012 Jan;4(1):83-101. doi: 10.3390/v4010083. Epub 2012 Jan 13. Viruses. 2012. PMID: 22355453 Free PMC article. Review.
-
Envelope glycoprotein of arenaviruses.Viruses. 2012 Oct 17;4(10):2162-81. doi: 10.3390/v4102162. Viruses. 2012. PMID: 23202458 Free PMC article. Review.
Cited by
-
Arenavirus budding: a common pathway with mechanistic differences.Viruses. 2013 Jan 31;5(2):528-49. doi: 10.3390/v5020528. Viruses. 2013. PMID: 23435234 Free PMC article. Review.
-
Old World arenavirus infection interferes with the expression of functional alpha-dystroglycan in the host cell.Mol Biol Cell. 2007 Nov;18(11):4493-507. doi: 10.1091/mbc.e07-04-0374. Epub 2007 Aug 29. Mol Biol Cell. 2007. PMID: 17761532 Free PMC article.
-
Junín Virus Promotes Autophagy To Facilitate the Virus Life Cycle.J Virol. 2019 Jul 17;93(15):e02307-18. doi: 10.1128/JVI.02307-18. Print 2019 Aug 1. J Virol. 2019. PMID: 31118257 Free PMC article.
-
Comparative analysis of disease pathogenesis and molecular mechanisms of New World and Old World arenavirus infections.J Gen Virol. 2014 Jan;95(Pt 1):1-15. doi: 10.1099/vir.0.057000-0. Epub 2013 Sep 25. J Gen Virol. 2014. PMID: 24068704 Free PMC article. Review.
-
Epidemiology and pathogenesis of Bolivian hemorrhagic fever.Curr Opin Virol. 2014 Apr;5:82-90. doi: 10.1016/j.coviro.2014.02.007. Epub 2014 Mar 15. Curr Opin Virol. 2014. PMID: 24636947 Free PMC article. Review.
References
-
- Albarino, C. G., P. D. Ghiringhelli, D. M. Posik, M. E. Lozano, A. M. Ambrosio, A. Sanchez, and V. Romanowski. 1997. Molecular characterization of attenuated Junin virus strains. J. Gen. Virol. 78:1605-1610. - PubMed
-
- Barrera Oro, J. G., and K. T. McKee, Jr. 1991. Toward a vaccine against Argentine hemorrhagic fever. Bull. Pan. Am. Health Organ. 25:118-126. - PubMed
-
- Binley, J. M., R. W. Sanders, B. Clas, N. Schuelke, A. Master, Y. Guo, F. Kajumo, P. J. Maddon, W. C. Olson, and J. P. Moore. 2000. A recombinant human immunodeficiency virus type 1 envelope glycoprotein complex stabilized by an intermolecular disulfide bond between gp120 and gp41 subunits is an antigenic mimic of the trimeric virion-associated structure. J. Virol. 74:627-643. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

