Mapping the Golgi targeting and retention signal of Bunyamwera virus glycoproteins
- PMID: 15367646
- PMCID: PMC516397
- DOI: 10.1128/JVI.78.19.10793-10802.2004
Mapping the Golgi targeting and retention signal of Bunyamwera virus glycoproteins
Abstract
The membrane glycoproteins (Gn and Gc) of Bunyamwera virus (BUN; family Bunyaviridae) accumulate in the Golgi complex, where virion maturation occurs. The Golgi targeting and retention signal has previously been shown to reside within the Gn protein. A series of truncated Gn and glycoprotein precursor cDNAs were constructed by progressively deleting the coding region of the transmembrane domain (TMD) and the cytoplasmic tail. We also constructed chimeric proteins of BUN Gc, enhanced green fluorescent protein (EGFP), and human respiratory syncytial virus (HRSV) fusion (F) protein that contain the Gn TMD with various lengths of its adjacent cytoplasmic tails. The subcellular localization of mutated BUN glycoproteins and chimeric proteins was investigated by double-staining immunofluorescence with antibodies against BUN glycoproteins or the HRSV F protein and with antibodies specific for the Golgi complex. The results revealed that Gn and all truncated Gn proteins that contained the intact TMD (residues 206 to 224) were able to translocate to the Golgi complex and also rescued the Gc protein, which is retained in the endoplasmic reticulum when expressed alone, to this organelle. The rescued Gc proteins acquired endo-beta-N-acetylglucosaminidase H resistance. The Gn TMD could also target chimeric EGFP to the Golgi and retain the F protein, which is characteristically expressed on the surface of HRSV-infected cells, in the Golgi. However, chimeric BUN Gc did not translocate to the Golgi, suggesting that an interaction with Gn is involved in Golgi retention of the Gc protein. Collectively, these data demonstrate that the Golgi targeting and retention signal of BUN glycoproteins resides in the TMD of the Gn protein.
Figures
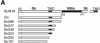
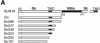






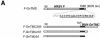
References
-
- Arkin, I. T. 2002. Structural aspects of oligomerization taking place between the transmembrane alpha-helices of bitopic membrane proteins. Biochim. Biophys. Acta 1565:347-363. - PubMed
-
- Bretscher, M. S., and S. Munro. 1993. Cholesterol and the Golgi apparatus. Science 261:1280-1281. - PubMed
-
- Chalfie, M., Y. Tu, G. Euskirchen, W. W. Ward, and D. C. Prasher. 1994. Green fluorescent protein as a marker for gene expression. Science 263:802-805. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

