Construction and characterization of a fluorescently labeled infectious human immunodeficiency virus type 1 derivative
- PMID: 15367647
- PMCID: PMC516407
- DOI: 10.1128/JVI.78.19.10803-10813.2004
Construction and characterization of a fluorescently labeled infectious human immunodeficiency virus type 1 derivative
Abstract
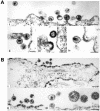
The introduction of a label which can be detected in living cells opens new possibilities for the direct analysis of dynamic processes in virus replication, such as the transport and assembly of structural proteins. Our aim was to generate a tool for the analysis of the trafficking of the main structural protein of human immunodeficiency virus type 1 (HIV-1), Gag, as well as for the analysis of virus-host cell interactions in an authentic setting. We describe here the construction and characterization of infectious HIV derivatives carrying a label within the Gag polyprotein. Based on our initial finding that a short epitope tag could be inserted near the C terminus of the matrix domain of Gag without affecting viral replication, we constructed HIV derivatives carrying the egfp gene at the analogous position, resulting in the expression of a Gag-EGFP fusion protein in the authentic viral context. Particles displaying normal viral protein compositions were released from transfected cells, and Gag-EGFP was efficiently processed by the viral protease, yielding the expected products. Furthermore, particles with mature morphology were observed by thin-section electron microscopy. The modified virus was even found to be infectious, albeit with reduced relative infectivity. By preparing mixed particles containing equimolar amounts of Gag-EGFP and Gag, we were able to obtain highly fluorescently labeled virion preparations which displayed normal morphology and full wild-type infectivity, demonstrating that the process of HIV particle assembly displays a remarkable flexibility. The fluorescent virus derivative is a useful tool for investigating the interaction of HIV with live cells.
Figures



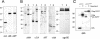

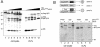

Similar articles
-
The role of nucleocapsid of HIV-1 in virus assembly.Virology. 1998 Nov 10;251(1):141-57. doi: 10.1006/viro.1998.9374. Virology. 1998. PMID: 9813210
-
Inhibition of viral assembly in murine cells by HIV-1 matrix.Virology. 2006 Aug 15;352(1):27-38. doi: 10.1016/j.virol.2006.04.024. Epub 2006 Jun 5. Virology. 2006. PMID: 16750235
-
Analysis of protein expression and virus-like particle formation in mammalian cell lines stably expressing HIV-1 gag and env gene products with or without active HIV proteinase.Virology. 1993 Feb;192(2):605-17. doi: 10.1006/viro.1993.1077. Virology. 1993. PMID: 8421902
-
HIV-1 gag proteins: diverse functions in the virus life cycle.Virology. 1998 Nov 10;251(1):1-15. doi: 10.1006/viro.1998.9398. Virology. 1998. PMID: 9813197 Review.
-
Functions of the HIV-1 matrix protein p17.New Microbiol. 2006 Jan;29(1):1-10. New Microbiol. 2006. PMID: 16608119 Review.
Cited by
-
Analysis of HIV-1 Gag protein interactions via biotin ligase tagging.J Virol. 2015 Apr;89(7):3988-4001. doi: 10.1128/JVI.03584-14. Epub 2015 Jan 28. J Virol. 2015. PMID: 25631074 Free PMC article.
-
Direct and dynamic detection of HIV-1 in living cells.PLoS One. 2012;7(11):e50026. doi: 10.1371/journal.pone.0050026. Epub 2012 Nov 28. PLoS One. 2012. PMID: 23209635 Free PMC article.
-
The HIV 5' Gag Region Displays a Specific Nucleotide Bias Regulating Viral Splicing and Infectivity.Viruses. 2021 May 27;13(6):997. doi: 10.3390/v13060997. Viruses. 2021. PMID: 34071819 Free PMC article.
-
HIV-1 assembly differentially alters dynamics and partitioning of tetraspanins and raft components.Traffic. 2010 Nov;11(11):1401-14. doi: 10.1111/j.1600-0854.2010.01111.x. Traffic. 2010. PMID: 20727121 Free PMC article.
-
Tetherin restricts productive HIV-1 cell-to-cell transmission.PLoS Pathog. 2010 Jun 17;6(6):e1000955. doi: 10.1371/journal.ppat.1000955. PLoS Pathog. 2010. PMID: 20585562 Free PMC article.
References
-
- Basyuk, E., T. Galli, M. Mougel, J. M. Blanchard, M. Sitbon, and E. Bertrand. 2003. Retroviral genomic RNAs are transported to the plasma membrane by endosomal vesicles. Dev. Cell 5:161-174. - PubMed
-
- Bohne, J., and H.-G. Kräusslich. 2004. Mutation of the major 5′ splice site renders a CMV-driven HIV-1 proviral clone Tat-dependent: connections between transcription and splicing. FEBS Lett. 563:113-118. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

