Swi1 and Swi3 are components of a replication fork protection complex in fission yeast
- PMID: 15367656
- PMCID: PMC516732
- DOI: 10.1128/MCB.24.19.8342-8355.2004
Swi1 and Swi3 are components of a replication fork protection complex in fission yeast
Abstract
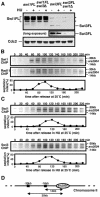
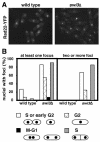
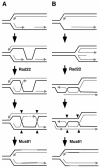
Swi1 is required for programmed pausing of replication forks near the mat1 locus in the fission yeast Schizosaccharomyces pombe. This fork pausing is required to initiate a recombination event that switches mating type. Swi1 is also needed for the replication checkpoint that arrests division in response to fork arrest. How Swi1 accomplishes these tasks is unknown. Here we report that Swi1 copurifies with a 181-amino-acid protein encoded by swi3(+). The Swi1-Swi3 complex is required for survival of fork arrest and for activation of the replication checkpoint kinase Cds1. Association of Swi1 and Swi3 with chromatin during DNA replication correlated with movement of the replication fork. swi1Delta and swi3Delta mutants accumulated Rad22 (Rad52 homolog) DNA repair foci during replication. These foci correlated with the Rad22-dependent appearance of Holliday junction (HJ)-like structures in cells lacking Mus81-Eme1 HJ resolvase. Rhp51 and Rhp54 homologous recombination proteins were not required for viability in swi1Delta or swi3Delta cells, indicating that the HJ-like structures arise from single-strand DNA gaps or rearranged forks instead of broken forks. We propose that Swi1 and Swi3 define a fork protection complex that coordinates leading- and lagging-strand synthesis and stabilizes stalled replication forks.
Figures



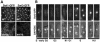

References
-
- Alcasabas, A. A., A. J. Osborn, J. Bachant, F. Hu, P. J. Werler, K. Bousset, K. Furuya, J. F. Diffley, A. M. Carr, and S. J. Elledge. 2001. Mrc1 transduces signals of DNA replication stress to activate Rad53. Nat. Cell Biol. 3:958-965. - PubMed
-
- Alfa, C., P. Fantes, J. Hyams, M. McLeod, and E. Warbrick. 1993. Experiments with fission yeast. Cold Spring Harbor Laboraotry Press, Cold Spring Harbor, N.Y.
-
- Bähler, J., J. Q. Wu, M. S. Longtine, N. G. Shah, A. McKenzie III, A. B. Steever, A. Wach, P. Philippsen, and J. R. Pringle. 1998. Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast 14:943-951. - PubMed
-
- Barnes, J. W., S. A. Tischkau, J. A. Barnes, J. W. Mitchell, P. W. Burgoon, J. R. Hickok, and M. U. Gillette. 2003. Requirement of mammalian Timeless for circadian rhythmicity. Science 302:439-442. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
