BRCA1-dependent ubiquitination of gamma-tubulin regulates centrosome number
- PMID: 15367667
- PMCID: PMC516733
- DOI: 10.1128/MCB.24.19.8457-8466.2004
BRCA1-dependent ubiquitination of gamma-tubulin regulates centrosome number
Abstract
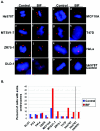
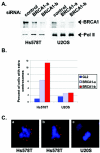
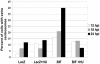
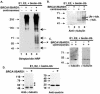
Proper centrosome duplication and spindle formation are crucial for prevention of chromosomal instability, and BRCA1 plays a role in this process. In this study, transient inhibition of BRCA1 function in cell lines derived from mammary tissue caused rapid amplification and fragmentation of centrosomes. Cell lines tested that were derived from nonmammary tissues did not amplify the centrosome number in this transient assay. We tested whether BRCA1 and its binding partner, BARD1, ubiquitinate centrosome proteins. Results showed that centrosome components, including gamma-tubulin, are ubiquitinated by BRCA1/BARD1 in vitro. The in vitro ubiquitination of gamma-tubulin was specific, and function of the carboxy terminus was necessary for this reaction; truncated BRCA1 did not ubiquitinate gamma-tubulin. BRCA1/BARD1 ubiquitinated lysines 48 and 344 of gamma-tubulin in vitro, and expression in cells of gamma-tubulin K48R caused a marked amplification of centrosomes. This result supports the notion that the modification of these lysines in living cells is critical in the maintenance of centrosome number. One of the key problems in understanding the biology of BRCA1 has been the identification of a specific target of BRCA1/BARD1 ubiquitination and its effect on mammary cell biology. The results of this study identify a ubiquitination target and suggest a biological impact important in the etiology of breast cancer.
Figures


Similar articles
-
Centrosomal microtubule nucleation activity is inhibited by BRCA1-dependent ubiquitination.Mol Cell Biol. 2005 Oct;25(19):8656-68. doi: 10.1128/MCB.25.19.8656-8668.2005. Mol Cell Biol. 2005. PMID: 16166645 Free PMC article.
-
Identification of domains of BRCA1 critical for the ubiquitin-dependent inhibition of centrosome function.Cancer Res. 2006 Apr 15;66(8):4100-7. doi: 10.1158/0008-5472.CAN-05-4430. Cancer Res. 2006. PMID: 16618730
-
Characterization of BRCA1 protein targeting, dynamics, and function at the centrosome: a role for the nuclear export signal, CRM1, and Aurora A kinase.J Biol Chem. 2012 Mar 2;287(10):7701-16. doi: 10.1074/jbc.M111.327296. Epub 2012 Jan 18. J Biol Chem. 2012. PMID: 22262852 Free PMC article.
-
The BRCA1-dependent ubiquitin ligase, gamma-tubulin, and centrosomes.Environ Mol Mutagen. 2009 Oct;50(8):649-53. doi: 10.1002/em.20475. Environ Mol Mutagen. 2009. PMID: 19274767 Review.
-
The Function of BARD1 in Centrosome Regulation in Cooperation with BRCA1/OLA1/RACK1.Genes (Basel). 2020 Jul 24;11(8):842. doi: 10.3390/genes11080842. Genes (Basel). 2020. PMID: 32722046 Free PMC article. Review.
Cited by
-
Drugging the addict: non-oncogene addiction as a target for cancer therapy.EMBO Rep. 2016 Nov;17(11):1516-1531. doi: 10.15252/embr.201643030. Epub 2016 Oct 4. EMBO Rep. 2016. PMID: 27702988 Free PMC article. Review.
-
Moonlighting in Mitosis: Analysis of the Mitotic Functions of Transcription and Splicing Factors.Cells. 2020 Jun 26;9(6):1554. doi: 10.3390/cells9061554. Cells. 2020. PMID: 32604778 Free PMC article. Review.
-
RAD6 inhibition enhances paclitaxel sensitivity of triple negative breast cancer cells by aggravating mitotic spindle damage.BMC Cancer. 2022 Oct 18;22(1):1073. doi: 10.1186/s12885-022-10119-z. BMC Cancer. 2022. PMID: 36258187 Free PMC article.
-
Genetic, functional, and histopathological evaluation of two C-terminal BRCA1 missense variants.J Med Genet. 2006 Jan;43(1):74-83. doi: 10.1136/jmg.2005.033258. Epub 2005 May 27. J Med Genet. 2006. PMID: 15923272 Free PMC article.
-
GC-MS Analysis, Molecular Docking and Pharmacokinetic Properties of Phytocompounds from Solanum torvum Unripe Fruits and Its Effect on Breast Cancer Target Protein.Appl Biochem Biotechnol. 2022 Jan;194(1):529-555. doi: 10.1007/s12010-021-03698-3. Epub 2021 Oct 13. Appl Biochem Biotechnol. 2022. PMID: 34643844 Free PMC article.
References
-
- Anderson, S. F., B. P. Schlegel, T. Nakajima, E. S. Wolpin, and J. D. Parvin. 1998. BRCA1 protein is linked to the RNA polymerase II holoenzyme complex via RNA helicase A. Nat. Genet. 19:254-256. - PubMed
-
- Bartek, J., J. Bartkova, N. Kyprianou, E. N. Lalani, Z. Staskova, M. Shearer, S. Chang, and J. Taylor-Papadimitriou. 1991. Efficient immortalization of luminal epithelial cells from human mammary gland by introduction of simian virus 40 large tumor antigen with a recombinant retrovirus. Proc. Natl. Acad. Sci. USA 88:3520-3524. - PMC - PubMed
-
- Berdichevsky, F., and J. Taylor-Papadimitriou. 1991. Morphological differentiation of hybrids of human mammary epithelial cell lines is dominant and correlates with the pattern of expression of intermediate filaments. Exp. Cell Res. 194:267-274. - PubMed
-
- Bornens, M., M. Paintrand, J. Berges, M. C. Marty, and E. Karsenti. 1987. Structural and chemical characterization of isolated centrosomes. Cell. Motil. Cytoskeleton 8:238-249. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
