Target gene-specific modulation of myocardin activity by GATA transcription factors
- PMID: 15367672
- PMCID: PMC516760
- DOI: 10.1128/MCB.24.19.8519-8528.2004
Target gene-specific modulation of myocardin activity by GATA transcription factors
Abstract
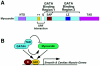
Myocardin is a transcriptional coactivator that regulates cardiac and smooth muscle gene expression by associating with serum response factor. We show that GATA transcription factors can either stimulate or suppress the transcriptional activity of myocardin, depending on the target gene. Modulation of myocardin activity by GATA4 is mediated by the physical interaction of myocardin with the DNA binding domain of GATA4 but does not require binding of GATA4 to DNA. Paradoxically, the transcription activation domain of GATA4 is dispensable for the stimulatory effect of GATA4 on myocardin activity but is required for repression of myocardin activity. The ability of GATA transcription factors to modulate myocardin activity provides a potential mechanism for fine tuning the expression of serum response factor target genes in a gene-specific manner.
Figures
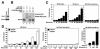
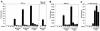



Similar articles
-
Myocardin is sufficient and necessary for cardiac gene expression in Xenopus.Development. 2005 Mar;132(5):987-97. doi: 10.1242/dev.01684. Epub 2005 Jan 26. Development. 2005. PMID: 15673566
-
Myocardin is a direct transcriptional target of Mef2, Tead and Foxo proteins during cardiovascular development.Development. 2006 Nov;133(21):4245-56. doi: 10.1242/dev.02610. Epub 2006 Oct 4. Development. 2006. PMID: 17021041
-
Activation of the cardiac alpha-actin promoter depends upon serum response factor, Tinman homologue, Nkx-2.5, and intact serum response elements.Dev Genet. 1996;19(2):119-30. doi: 10.1002/(SICI)1520-6408(1996)19:2<119::AID-DVG3>3.0.CO;2-C. Dev Genet. 1996. PMID: 8900044
-
GATA transcription factors and cardiac development.Semin Cell Dev Biol. 1999 Feb;10(1):85-91. doi: 10.1006/scdb.1998.0281. Semin Cell Dev Biol. 1999. PMID: 10355032 Review.
-
Regulation of cardiac myocyte cell death and differentiation by myocardin.Mol Cell Biochem. 2018 Jan;437(1-2):119-131. doi: 10.1007/s11010-017-3100-3. Epub 2017 Jun 19. Mol Cell Biochem. 2018. PMID: 28631251 Review.
Cited by
-
Smooth muscle-specific genes are differentially sensitive to inhibition by Elk-1.Mol Cell Biol. 2005 Nov;25(22):9874-85. doi: 10.1128/MCB.25.22.9874-9885.2005. Mol Cell Biol. 2005. PMID: 16260603 Free PMC article.
-
Re-employment of developmental transcription factors in adult heart disease.Semin Cell Dev Biol. 2007 Feb;18(1):117-31. doi: 10.1016/j.semcdb.2006.11.012. Epub 2006 Nov 24. Semin Cell Dev Biol. 2007. PMID: 17161634 Free PMC article. Review.
-
Myocardin regulates mitochondrial calcium homeostasis and prevents permeability transition.Cell Death Differ. 2018 Nov;25(10):1732-1748. doi: 10.1038/s41418-018-0073-z. Epub 2018 Mar 6. Cell Death Differ. 2018. PMID: 29511336 Free PMC article.
-
Myocardin in biology and disease.J Biomed Res. 2015 Jan;29(1):3-19. doi: 10.7555/JBR.29.20140151. Epub 2014 Dec 25. J Biomed Res. 2015. PMID: 25745471 Free PMC article. Review.
-
GATA-6 and NF-κB activate CPI-17 gene transcription and regulate Ca2+ sensitization of smooth muscle contraction.Mol Cell Biol. 2013 Mar;33(5):1085-102. doi: 10.1128/MCB.00626-12. Epub 2012 Dec 28. Mol Cell Biol. 2013. PMID: 23275439 Free PMC article.
References
-
- Aravind, L., and E. V. Koonin. 2000. SAP—a putative DNA-binding motif involved in chromosomal organization. Trends Biochem. Sci. 25:112-114. - PubMed
-
- Chang, P. S., L. Li, J. McAnally, and E. N. Olson. 2001. Muscle specificity encoded by specific serum response factor-binding sites. J. Biol. Chem. 276:17206-17212. - PubMed
-
- Chang, D. F., N. S. Belaguli, D. Iyer, W. B. Roberts, S. P. Wu, X. R. Dong, J. G. Marx, M. S. Moore, M. C. Beckerle, M. W. Majesky, and R. J. Schwartz. 2003. Cysteine-rich LIM-only proteins CRP1 and CRP2 are potent smooth muscle differentiation cofactors. Dev. Cell. 4:107-118. - PubMed
-
- Charron, F., and M. Nemer. 1999. GATA transcription factors and cardiac development. Semin. Cell Dev. Biol. 10:85-91. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
