Loss of function but no gain of function caused by amino acid substitutions in the hexapeptide of Hoxa1 in vivo
- PMID: 15367676
- PMCID: PMC516739
- DOI: 10.1128/MCB.24.19.8567-8575.2004
Loss of function but no gain of function caused by amino acid substitutions in the hexapeptide of Hoxa1 in vivo
Abstract
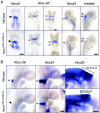
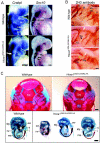
Homeodomain containing transcription factors of the Hox family play critical roles in patterning the anteroposterior embryonic body axis, as well as in controlling several steps of organogenesis. Several Hox proteins have been shown to cooperate with members of the Pbx family for the recognition and activation of identified target enhancers. Hox proteins contact Pbx via a conserved hexapeptide motif. Previous biochemical studies provided evidence that critical amino acid substitutions in the hexapeptide sequence of Hoxa1 abolish its interaction with Pbx. As a result, these substitutions also abolish Hoxa1 activity on known target enhancers in cellular models, suggesting that Hoxa1 activity relies on its capacity to interact with Pbx. Here, we show that mice with mutations in the Hoxa1 hexapeptide display hindbrain, cranial nerve, and skeletal defects highly reminiscent of those reported for the Hoxa1 loss of function. Since similar hexapeptide mutations in the mouse Hoxb8 and the Drosophila AbdA proteins result in activity modulation and gain of function, our data demonstrate that the functional importance of the hexapeptide in vivo differs according to the Hox proteins.
Figures


References
-
- Barrow, J. R., and M. R. Capecchi. 1999. Compensatory defects associated with mutations in Hoxa1 restore normal palatogenesis to Hoxa2 mutants. Development 126:5011-5026. - PubMed
-
- Capecchi, M. R. 1997. Hox genes and mammalian development. Cold Spring Harbor Symp. Quant. Biol. 62:273-281. - PubMed
-
- Carpenter, E. M., G. M. Goddard, O. Chisaka, N. R. Manley, and M. R. Capecchi. 1993. Loss of Hoxa-1 (Hox-1.6) function results in the reorganization of the murine hindbrain. Development 118:1063-1075. - PubMed
-
- Chan, S.-K., L. Jaffe, M. Capovilla, J. Botas, and R. Mann. 1994. The DNA binding specificity of Ultrabithorax is modulated by cooperative interactions with extradenticle, another homeoprotein. Cell 78:603-615. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
