Early activation of sphingosine kinase in mast cells and recruitment to FcepsilonRI are mediated by its interaction with Lyn kinase
- PMID: 15367693
- PMCID: PMC516729
- DOI: 10.1128/MCB.24.19.8765-8777.2004
Early activation of sphingosine kinase in mast cells and recruitment to FcepsilonRI are mediated by its interaction with Lyn kinase
Abstract
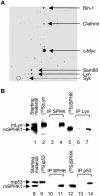
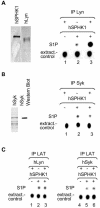
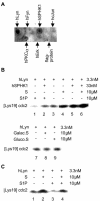
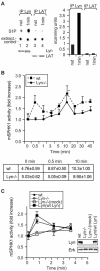
Sphingosine kinase has been recognized as an essential signaling molecule that mediates the intracellular conversion of sphingosine to sphingosine-1-phosphate. In mast cells, induction of sphingosine kinase and generation of sphingosine-1-phosphate have been linked to the initial rise in Ca(2+), released from internal stores, and to degranulation. These events either precede or are concomitant with the activation of phospholipase C-gamma and the generation of inositol trisphosphate. Here we show that sphingosine kinase type 1 (SPHK1) interacts directly with the tyrosine kinase Lyn and that this interaction leads to the recruitment of this lipid kinase to the high-affinity receptor for immunoglobulin E (FcepsilonRI). The interaction of SPHK1 with Lyn caused enhanced lipid and tyrosine kinase activity. After FcepsilonRI triggering, enhanced sphingosine kinase activity was associated with FcepsilonRI in sphingolipid-enriched rafts of mast cells. Bone marrow-derived mast cells from Lyn(-/)(-) mice, compared to syngeneic wild-type cells, were defective in the initial induction of SPHK1 activity, and the defect was overcome by retroviral Lyn expression. These findings position the activation of SPHK1 as an FcepsilonRI proximal event.
Figures




References
-
- Albrecht, B., M. Woisetschlager, and M. W. Robertson. 2000. Export of the high affinity IgE receptor from the endoplasmic reticulum depends on a glycosylation-mediated quality control mechanism. J. Immunol. 165:5686-5694. - PubMed
-
- Baumruker, T., G. G. Pendl, and E. E. Prieschl. 1997. Gene regulation after Fc epsilon RI stimulation in the murine mast cell line CPII. Int. Arch. Allergy Immunol. 113:39-41. - PubMed
-
- Baumruker, T., and E. E. Prieschl. 2002. Sphingolipids and the regulation of the immune response. Semin. Immunol. 14:57-63. - PubMed
-
- Billich, A., F. Bornancin, P. Devay, D. Mechtcheriakova, N. Urtz, and T. Baumruker. 2003. Phosphorylation of the immunomodulatory drug FTY720 by sphingosine kinases. J. Biol. Chem. 278:47408-47415. - PubMed
-
- Brinkmann, V., M. D. Davis, C. E. Heise, R. Albert, S. Cottens, R. Hof, C. Bruns, E. Prieschl, T. Baumruker, P. Hiestand, C. A. Foster, M. Zollinger, and K. R. Lynch. 2002. The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J. Biol. Chem. 277:21453-21457. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
