Protein phosphatase 2A negatively regulates insulin's metabolic signaling pathway by inhibiting Akt (protein kinase B) activity in 3T3-L1 adipocytes
- PMID: 15367694
- PMCID: PMC516764
- DOI: 10.1128/MCB.24.19.8778-8789.2004
Protein phosphatase 2A negatively regulates insulin's metabolic signaling pathway by inhibiting Akt (protein kinase B) activity in 3T3-L1 adipocytes
Abstract
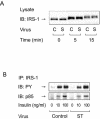
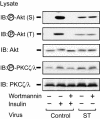
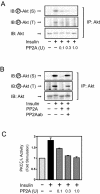
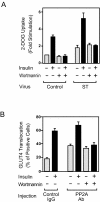
Protein phosphatase 2A (PP2A) is a multimeric serine/threonine phosphatase which has multiple functions, including inhibition of the mitogen-activated protein (MAP) kinase pathway. Simian virus 40 small t antigen specifically inhibits PP2A function by binding to the PP2A regulatory subunit, interfering with the ability of PP2A to associate with its cellular substrates. We have reported that the expression of small t antigen inhibits PP2A association with Shc, leading to augmentation of insulin and epidermal growth factor-induced Shc phosphorylation with enhanced activation of the Ras/MAP kinase pathway. However, the potential involvement of PP2A in insulin's metabolic signaling pathway is presently unknown. To assess this, we overexpressed small t antigen in 3T3-L1 adipocytes by adenovirus-mediated gene transfer and found that the phosphorylation of Akt and its downstream target, glycogen synthase kinase 3beta, were enhanced both in the absence and in the presence of insulin. Furthermore, protein kinase C lambda (PKC lambda) activity was also augmented in small-t-antigen-expressing 3T3-L1 adipocytes. Consistent with this result, both basal and insulin-stimulated glucose uptake were enhanced in these cells. In support of this result, when inhibitory anti-PP2A antibody was microinjected into 3T3-L1 adipocytes, we found a twofold increase in GLUT4 translocation in the absence of insulin. The small-t-antigen-induced increase in Akt and PKC lambda activities was not inhibited by wortmannin, while the ability of small t antigen to enhance glucose transport was inhibited by dominant negative Akt (DN-Akt) expression and Akt small interfering RNA (siRNA) but not by DN-PKC lambda expression or PKC lambda siRNA. We conclude that PP2A is a negative regulator of insulin's metabolic signaling pathway by promoting dephosphorylation and inactivation of Akt and PKC lambda and that most of the effects of PP2A to inhibit glucose transport are mediated through Akt.
Figures






Similar articles
-
Protein phosphatase 2A forms a molecular complex with Shc and regulates Shc tyrosine phosphorylation and downstream mitogenic signaling.Mol Cell Biol. 2002 Apr;22(7):2375-87. doi: 10.1128/MCB.22.7.2375-2387.2002. Mol Cell Biol. 2002. PMID: 11884620 Free PMC article.
-
Activation of the mammalian target of rapamycin pathway acutely inhibits insulin signaling to Akt and glucose transport in 3T3-L1 and human adipocytes.Endocrinology. 2005 Mar;146(3):1328-37. doi: 10.1210/en.2004-0777. Epub 2004 Dec 2. Endocrinology. 2005. PMID: 15576463
-
Overexpression of SH2-containing inositol phosphatase 2 results in negative regulation of insulin-induced metabolic actions in 3T3-L1 adipocytes via its 5'-phosphatase catalytic activity.Mol Cell Biol. 2001 Mar;21(5):1633-46. doi: 10.1128/MCB.21.5.1633-1646.2001. Mol Cell Biol. 2001. PMID: 11238900 Free PMC article.
-
Protein phosphatase-1 and insulin action.Mol Cell Biochem. 1998 May;182(1-2):49-58. Mol Cell Biochem. 1998. PMID: 9609113 Review.
-
Human Protein Kinases and Obesity.Adv Exp Med Biol. 2017;960:111-134. doi: 10.1007/978-3-319-48382-5_5. Adv Exp Med Biol. 2017. PMID: 28585197 Review.
Cited by
-
Unexpected severe consequences of Pikfyve deletion by aP2- or Aq-promoter-driven Cre expression for glucose homeostasis and mammary gland development.Physiol Rep. 2016 Jun;4(11):e12812. doi: 10.14814/phy2.12812. Physiol Rep. 2016. PMID: 27273882 Free PMC article.
-
DAF-16/Forkhead box O transcription factor: many paths to a single Fork(head) in the road.Antioxid Redox Signal. 2011 Feb 15;14(4):623-34. doi: 10.1089/ars.2010.3490. Epub 2010 Nov 30. Antioxid Redox Signal. 2011. PMID: 20673162 Free PMC article.
-
Phosphatase inhibition by sodium orthovanadate displays anti-inflammatory action by suppressing AKT-IKKβ signaling in RAW264.7 cells.Toxicol Rep. 2022 Oct 7;9:1883-1893. doi: 10.1016/j.toxrep.2022.09.012. eCollection 2022. Toxicol Rep. 2022. PMID: 36518371 Free PMC article.
-
Angiotensin II Inhibits Insulin Receptor Signaling in Adipose Cells.Int J Mol Sci. 2022 May 27;23(11):6048. doi: 10.3390/ijms23116048. Int J Mol Sci. 2022. PMID: 35682723 Free PMC article.
-
Decreased gene expressions of insulin signal molecules in canine hyperadrenocorticism.J Vet Med Sci. 2014 Aug;76(8):1177-82. doi: 10.1292/jvms.14-0033. Epub 2014 May 15. J Vet Med Sci. 2014. PMID: 24829079 Free PMC article.
References
-
- Abraham, D., K. Podar, M. Pacher, M. Kubicek, N. Welzel, B. A. Hemmings, S. M. Dilworth, H. Mischak, W. Kolch, and M. Baccarini. 2000. Raf-1-associated protein phosphatase 2A as a positive regulator of kinase activation. J. Biol. Chem. 275:22300-22304. - PubMed
-
- Aguirre, V., E. D. Werner, J. Giraud, Y. H. Lee, S. E. Shoelson, and M. F. White. 2002. Phosphorylation of Ser307 in insulin receptor substrate-1 blocks interactions with the insulin receptor and inhibits insulin action. J. Biol. Chem. 277:1531-1537. - PubMed
-
- Ahmad, F., R. V. Considine, T. L. Bauer, J. P. Ohannesian, C. C. Marco, and B. J. Goldstein. 1997. Improved sensitivity to insulin in obese subjects following weight loss is accompanied by reduced protein-tyrosine phosphatases in adipose tissue. Metabolism 46:1140-1145. - PubMed
-
- Ahmad, F., and B. J. Goldstein. 1995. Increased abundance of specific skeletal muscle protein-tyrosine phosphatases in a genetic model of insulin-resistant obesity and diabetes mellitus. Metabolism 44:1175-1184. - PubMed
-
- Alessi, D. R., N. Gomez, G. Moorhead, T. Lewis, S. M. Keyse, and P. Cohen. 1995. Inactivation of p42 MAP kinase by protein phosphatase 2A and a protein tyrosine phosphatase, but not CL100, in various cell lines. Curr. Biol. 5:283-295. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
