Glucagon-like peptide 1 excites hypocretin/orexin neurons by direct and indirect mechanisms: implications for viscera-mediated arousal
- PMID: 15371515
- PMCID: PMC6729787
- DOI: 10.1523/JNEUROSCI.1607-04.2004
Glucagon-like peptide 1 excites hypocretin/orexin neurons by direct and indirect mechanisms: implications for viscera-mediated arousal
Abstract
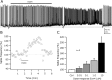
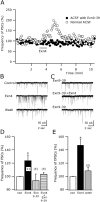
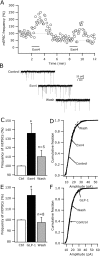
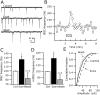
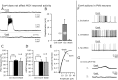
Glucagon-like peptide 1 (GLP-1) is produced by neurons in the caudal brainstem that receive sensory information from the gut and project to several hypothalamic regions involved in arousal, interoceptive stress, and energy homeostasis. GLP-1 axons and receptors have been detected in the lateral hypothalamus, where hypocretin neurons are found. The electrophysiological actions of GLP-1 in the CNS have not been studied. Here, we explored the GLP-1 effects on GFP (green fluorescent protein)-expressing hypocretin neurons in mouse hypothalamic slices. GLP-1 receptor agonists depolarized hypocretin neurons and increased their spike frequency; the antagonist exendin (9-39) blocked this depolarization. Direct GLP-1 agonist actions on membrane potential were abolished by choline substitution for extracellular Na+, and dependent on intracellular GDP, suggesting that they were mediated by sodium-dependent conductances in a G-protein-dependent manner. In voltage clamp, the GLP-1 agonist Exn4 (exendin-4) induced an inward current that reversed near -28 mV and persisted in nominally Ca2+-free extracellular solution, consistent with a nonselective cationic conductance. GLP-1 decreased afterhyperpolarization currents. GLP-1 agonists enhanced the frequency of miniature and spontaneous EPSCs with no effect on their amplitude, suggesting presynaptic modulation of glutamate axons innervating hypocretin neurons. Paraventricular hypothalamic neurons were also directly excited by GLP-1 agonists. In contrast, GLP-1 agonists had no detectable effect on neurons that synthesize melanin-concentrating hormone (MCH). Together, our results show that GLP-1 agonists modulate the activity of hypocretin, but not MCH, neurons in the lateral hypothalamus, suggesting a role for GLP-1 in the excitation of the hypothalamic arousal system possibly initiated by activation by viscera sensory input.
Figures


References
-
- Bekkers JM, Stevens CF (1995) Quantal analysis of EPSCs recorded from small numbers of synapses in hippocampal cultures. J Neurophysiol 73: 1145-1156. - PubMed
-
- Broberger C, de Lecea L, Sutcliffe JG, Hökfelt T (1998) Hypocretin/orexin and melanin-concentrating hormone-expressing cells form distinct populations in the rodent lateral hypothalamus: relationship to the neuropeptide Y and Agouti gene-related protein systems. J Comp Neurol 402: 460-474. - PubMed
-
- Borowsky B, Durkin MM, Ogozalek K, Marzabadi MR, DeLeon J, Lagu B, Heurich R, Lichtblau H, Shaposhnik Z, Daniewska I, Blackburn TP, Branchek TA, Gerald C, Vaysse PJ, Forray C (2002) Antidepressant, anxiolytic and anorectic effects of a melanin-concentrating hormone-1 receptor antagonist. Nat Med 8: 825-830. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
