Apatite-mediated actin dynamics in resorbing osteoclasts
- PMID: 15371537
- PMCID: PMC532006
- DOI: 10.1091/mbc.e04-06-0522
Apatite-mediated actin dynamics in resorbing osteoclasts
Abstract
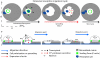
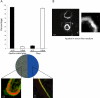
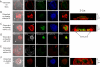
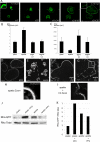
The actin cytoskeleton is essential for osteoclasts main function, bone resorption. Two different organizations of actin have been described in osteoclasts, the podosomes belt corresponding to numerous F-actin columns arranged at the cell periphery, and the sealing zone defined as a unique large band of actin. To compare the role of these two different actin organizations, we imaged osteoclasts on various substrata: glass, dentin, and apatite. Using primary osteoclasts expressing GFP-actin, we found that podosome belts and sealing zones, both very dynamic actin structures, were present in mature osteoclasts; podosome belts were observed only in spread osteoclasts adhering onto glass, whereas sealing zone were seen in apico-basal polarized osteoclasts adherent on mineralized matrix. Dynamic observations of several resorption cycles of osteoclasts seeded on apatite revealed that 1) podosomes do not fuse together to form the sealing zone; 2) osteoclasts alternate successive stationary polarized resorption phases with a sealing zone and migration, nonresorption phases without any specific actin structure; and 3) apatite itself promotes sealing zone formation though c-src and Rho signaling. Finally, our work suggests that apatite-mediated sealing zone formation is dependent on both c-src and Rho whereas apico-basal polarization requires only Rho.
Figures



References
-
- Bourette, R.P., Mouchiroud, G., Ouazana, R., Morle, F., Godet, J., and Blanchet, J.P. (1993). Expression of human colony-stimulating factor-1 (CSF-1) receptor in murine pluripotent hematopoietic NFS-60 cells induces long-term proliferation in response to CSF-1 without loss of erythroid differentiation potential. Blood 81, 2511–2520. - PubMed
-
- Chambers, T.J., Thomson, B.M., and Fuller, K. (1984). Effect of substrate composition on bone resorption by rabbit osteoclasts. J. Cell Sci. 70, 61–71. - PubMed
-
- Chellaiah, M.A., Biswas, R.S., Rittling, S.R., Denhardt, D.T., and Hruska, K.A. (2003a). Rho-dependent Rho Kinase activation increases CD44 surface expression and bone resorption in osteoclasts. J. Biol. Chem. 278, 29086–29097. - PubMed
-
- Chellaiah, M.A., Soga, N., Swanson, S., McAllister, S., Alvarez, U., Wang, D., Dowdy, S.F., and Hruska, K.A. (2000). Rho-A is critical for osteoclast podosome organization, motility, and bone resorption. J. Biol. Chem. 275, 11993–12002. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

