Trophoblast differentiation during embryo implantation and formation of the maternal-fetal interface
- PMID: 15372095
- PMCID: PMC516273
- DOI: 10.1172/JCI22991
Trophoblast differentiation during embryo implantation and formation of the maternal-fetal interface
Abstract
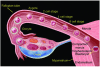
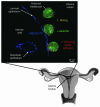
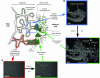
Trophoblasts, the specialized cells of the placenta, play a major role in implantation and formation of the maternal-fetal interface. Through an unusual differentiation process examined in this review, these fetal cells acquire properties of leukocytes and endothelial cells that enable many of their specialized functions. In recent years a great deal has been learned about the regulatory mechanisms, from transcriptional networks to oxygen tension, which control trophoblast differentiation. The challenge is to turn this information into clinically useful tests for monitoring placental function and, hence, pregnancy outcome.
Figures


References
-
- Wu L, et al. Extra-embryonic function of Rb is essential for embryonic development and viability. Nature. 2003;421:942–947. - PubMed
-
- Sibai BM, et al. Risk factors associated with preeclampsia in healthy nulliparous women. The Calcium for Preeclampsia Prevention (CPEP) Study Group. Am. J. Obstet. Gynecol. 1997;177:1003–1010. - PubMed
-
- Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N. Engl. J. Med. 2000;342:1500–1507. - PubMed
-
- Kunath T, Strumpf D, Rossant J. Early trophoblast determination and stem cell maintenance in the mouse-a review. Placenta. 2004;25(Suppl.):S32–S38. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

