The renal papilla is a niche for adult kidney stem cells
- PMID: 15372103
- PMCID: PMC516259
- DOI: 10.1172/JCI20921
The renal papilla is a niche for adult kidney stem cells
Abstract
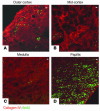
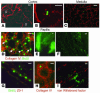
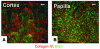
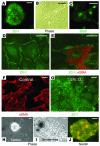
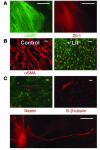
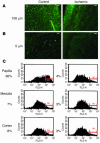
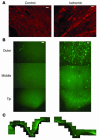
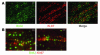
Many adult organs contain stem cells, which are pluripotent and are involved in organ maintenance and repair after injury. In situ, these cells often have a low cycling rate and locate in specialized regions (niches). To detect such cells in the kidney, we administered a pulse of the nucleotide bromodeoxyuridine (BrdU) to rat and mouse pups and, after a long (more than 2-month) chase, examined whether the kidney contained a population of low-cycling cells. We found that in the adult kidney, BrdU-retaining cells were very sparse except in the renal papilla, where they were numerous. During the repair phase of transient renal ischemia, these cells entered the cell cycle and the BrdU signal quickly disappeared from the papilla, despite the absence of apoptosis in this part of the kidney. In vitro isolation of renal papillary cells showed them to have a plastic phenotype that could be modulated by oxygen tension and that when injected into the renal cortex, they incorporated into the renal parenchyma. In addition, like other stem cells, papillary cells spontaneously formed spheres. Single-cell clones of these cells coexpressed mesenchymal and epithelial proteins and gave rise to myofibroblasts, cells expressing neuronal markers, and cells of uncharacterized phenotype. These data indicate that the renal papilla is a niche for adult kidney stem cells.
Figures

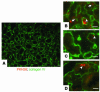
References
-
- Witzgall R, Brown D, Schwarz C, Bonventre JV. Localization of proliferating cell nuclear antigen, vimentin, c-Fos, and clusterin in the postischemic kidney. Evidence for a heterogenous genetic response among nephron segments, and a large pool of mitotically active and dedifferentiated cells. J. Clin. Invest. 1994;93:2175–2188. - PMC - PubMed
-
- Finn WF, Chevalier RL. Recovery from postischemic acute renal failure in the rat. Kidney Int. 1979;16:113–123. - PubMed
-
- Gupta S, Verfaillie C, Chmielewski D, Kim Y, Rosenberg ME. A role for extrarenal cells in the regeneration following acute renal failure. Kidney Int. 2002;62:1285–1290. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

