Comparison of bronchoprotective and bronchodilator effects of a single dose of formoterol delivered by hydrofluoroalkane and chlorofluorocarbon aerosols and dry powder in a double blind, placebo-controlled, crossover study
- PMID: 15373928
- PMCID: PMC1884606
- DOI: 10.1111/j.1365-2125.2004.02172.x
Comparison of bronchoprotective and bronchodilator effects of a single dose of formoterol delivered by hydrofluoroalkane and chlorofluorocarbon aerosols and dry powder in a double blind, placebo-controlled, crossover study
Abstract
Background: In response to the phasing out of chlorofluorocarbon (CFC) inhalers, a metered dose hydrofluoroalkane (HFA) formulation, Modulite (Chiesi Farmaceutici S.p.A, Parma, Italy), to be delivered with a pressurized metered dose inhaler (pMDI), has been developed. Modulite is a HFA formulation technology that has been designed to provide stable and uniform dose delivery of HFA-based formulations to enable an easy transition from CFC to HFA inhalers.
Objectives: The aim of this study was to compare the bronchoprotective and bronchodilator effects of a single dose of 12 microg of formoterol from the HFA Modulite inhaler with the Foradil Aerolizer (dry powder inhaler, DPI) and the Foradil CFC inhalers (Novartis Health Consumer, Basel, Switzerland).
Methods: This was a double blind, double dummy, randomized, placebo-controlled, crossover study conducted in 38 subjects with mild to moderate asthma (mean forced expiratory volume in 1 s [FEV1] 87.5% predicted). The primary endpoint was methacholine challenge provocative dose required for 20% fall in the FEV1 (PD20) 90 min post dose. Bronchodilation was assessed with spirometry (FEV1, FVC, FEF25-75) and impulse oscillometry (resistance at 5 and 20 Hz, reactance at 5 Hz and resonant frequency) over the 90 min post dose. In a subset of 12 subjects formoterol plasma levels, serum potassium and glucose were determined up to 480 min post dose.
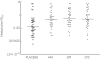
Results: The three formoterol formulations demonstrated significant (P < or = 0.05) improvements in bronchoprotection compared to placebo and non-inferiority of the HFA preparation compared to the CFC and DPI preparations was demonstrated. Geometric mean PD20 values were 0.51 mg with HFA, 0.62 mg with DPI, 0.62 mg with CFC and 0.2 mg with placebo. The log transformed mean differences in PD20 doubling dose between HFA and (a) DPI was -0.28 (95% CI -0.84-0.29, P = 0.57) (b) CFC was -0.28 (95% CI -0.84-0.28, P = 0.57) and (c) placebo was 1.38 (95% CI 0.82-1.94, P < 0.001). Serum potassium, glucose and formoterol plasma profiles were comparable for the CFC, HFA and DPI devices.
Conclusion: Our findings of similar efficacy, pharmacokinetics and systemic effects of the HFA formoterol inhaler compared to the CFC and DPI preparations supports the potential use of this novel formulation in the treatment of asthma.
Copyright 2004 Blackwell Publishing Ltd
Figures


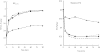

References
-
- United Nations Environmental Programme (Ozone Secretariat) Handbook for the International Treaties for the Protection of the Ozone Layer. 4. Kenya: 1996.
-
- Kleerup EC, Tashkin DP, Cline AC, Ekholm BP. Cumulative Dose-Response Study of Non-CFC Propellant HFA 134a Salbutamol Sulfate Metered-Dose Inhaler in Patients with Asthma. Chest. 1996;109:702–7. - PubMed
-
- Langley SJ, Sykes AP, Batty EP, Masterson CM, Woodcock AA. A Comparison of Efficacy and Tolerability of Single Doses of HFA 134a Albuterol and CFC Albuterol in mild –to-moderate asthmatic patients. Ann Allergy Asthma Immunol. 2002;88:488–93. - PubMed
-
- Langley SJ, Holden J, Derham A, Hedgeland P, Sharma RK, Woodcock AA. Fluticasone Propionate via the Diskhaler or Hydrofluoroalkane-134a Metered- Dose inhaler on Methacholine-Induced Airway Hperresponsiveness. Chest. 2002;122:806–11. - PubMed
-
- Woodcock A, Williams A, Batty L, Masterson C, Rossetti A, Cantini L. Effects on Lung Function, Symptoms, and Bronchial Hyperreactivity of low-dose inhaled beclomethasone dipropionate given with HFA-134a or CFC propellant. J Aerosol Med. 2002;15(4):407–14. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

