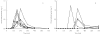Pharmacokinetics of oral fumarates in healthy subjects
- PMID: 15373936
- PMCID: PMC1884599
- DOI: 10.1111/j.1365-2125.2004.02145.x
Pharmacokinetics of oral fumarates in healthy subjects
Abstract
Aims: To characterize the pharmacokinetics of fumarates in healthy subjects.
Methods: Ten subjects received a single fumarate tablet (containing 120 mg of dimethylfumarate and 95 mg of calcium-monoethylfumarate) in the fasted state and after a standardized breakfast in randomized order. Prior to and at fixed intervals after the dose, blood samples were drawn and the concentrations of monomethylfumarate, the biologically active metabolite, as well as dimethylfumarate and fumaric acid were measured using high-performance liquid chromatography.
Results: After a lag time, a transient increase in serum monomethylfumarate concentrations in the blood was observed, whereas dimethylfumarate and fumaric acid concentrations remained below the detection limit. The tlag was 240 min [range 60-603 min; 95% confidence interval (CI) 139, 471] shorter when the tablet was taken after an overnight fast (90 min; range 60-120 min; 95% CI 66, 107) than when taken with breakfast (300 min; range 180-723 min; 95% CI 0, 1002). The tmax was 241 min (range 60-1189 min, 95% CI 53, 781) shorter when the tablet was taken after an overnight fast (182 min; range 120-240 min; 95% CI 146, 211) than when taken with breakfast (361 min; range 240-1429 min; 95% CI 0, 1062). The mean Cmax for monomethylfumarate in the blood of fasting subjects was to 0.84 mg l(-1) (range 0.37-1.29 mg l(-1); 95% CI 0.52, 1.07) and did not differ from that in fed subjects (0.48 mg l(-1); range 0-1.22 mg l(-1); 95% CI 0, 5.55).
Conclusions: The pharmacokinetics of monomethylfumarate in healthy subjects after a single tablet of fumarate are highly variable, particularly after food intake. Further experiments exploring the pharmacokinetics of oral fumarates are warranted in order to elucidate the mechanisms underlying variability in response in patients.
Copyright 2004 Blackwell Publishing Ltd
Figures
References
-
- Kroesen S, Widmer AF, Tyndall A, Hasler P. Serious bacterial infections in patients with rheumatoid arthritis under anti-TNF-alpha therapy. Rheumatology (Oxford) 2003;42:617–21. - PubMed
-
- Davison SC, Bunker CB, Basarab T. Etanercept for severe psoriasis and psoriatic arthritis: observations on combination therapy. Br J Dermatol. 2002;147:831–2. - PubMed
-
- Koo J, Lee J. Cyclosporine: what clinicians need to know. Dermatol Clin. 1995;13:897–907. - PubMed
-
- Griffiths CE, Clark CM, Chalmers RJ, Li Wan PA, Williams HC. A systematic review of treatments for severe psoriasis. Health Technol Assess. 2000;4:1–125. - PubMed
-
- Lebwohl M. Psoriasis. Lancet. 2003;361:1197–204. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources