Transcriptional regulation of Vibrio cholerae hemagglutinin/protease by the cyclic AMP receptor protein and RpoS
- PMID: 15375117
- PMCID: PMC516606
- DOI: 10.1128/JB.186.19.6374-6382.2004
Transcriptional regulation of Vibrio cholerae hemagglutinin/protease by the cyclic AMP receptor protein and RpoS
Abstract
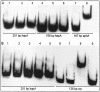
Vibrio cholerae secretes a Zn-dependent metalloprotease, hemagglutinin/protease (HA/protease), which is encoded by hapA and displays a broad range of potentially pathogenic activities. Production of HA/protease requires transcriptional activation by the quorum-sensing regulator HapR. In this study we demonstrate that transcription of hapA is growth phase dependent and specifically activated in the deceleration and stationary growth phases. Addition of glucose in these phases repressed hapA transcription by inducing V. cholerae to resume exponential growth, which in turn diminished the expression of a rpoS-lacZ transcriptional fusion. Contrary to a previous observation, we demonstrate that transcription of hapA requires the rpoS-encoded sigma(s) factor. The cyclic AMP (cAMP) receptor protein (CRP) strongly enhanced hapA transcription in the deceleration phase. Analysis of rpoS and hapR mRNA in isogenic CRP+ and CRP- strains suggested that CRP enhances the transcription of rpoS and hapR. Analysis of strains containing hapR-lacZ and hapA-lacZ fusions confirmed that hapA is transcribed in response to concurrent quorum-sensing and nutrient limitation stimuli. Mutations inactivating the stringent response regulator RelA and the HapR-controlled AphA regulator did not affect HA/protease expression. Electrophoretic mobility shift experiments showed that pure cAMP-CRP and HapR alone do not bind the hapA promoter. This result suggests that HapR activation of hapA differs from its interaction with the aphA promoter and could involve additional factors.
Figures
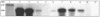


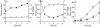



References
-
- Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1995. Short protocols in molecular biology. John Wiley & Sons Inc., New York, N.Y.
-
- Benitez, J. A., L. Garcia, A. J. Silva, H. Garcia, R. Fando, B. Cedre, A. Perez, J. Campos, B. L. Rodriguez, J. L. Perez, T. Valmaseda, O. Perez, A. Perez, M. Ramirez, T. Ledon, M. Diaz, M. Lastre, L. Bravo, and G. Sierra. 1999. Preliminary assessment of the safety and immunogenicity of a new CTXΦ-negative hemagglutinin/protease-defective E1 Tor strain as a cholera vaccine candidate. Infect. Immun. 67:539-545. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

