Bacillus subtilis ResD induces expression of the potential regulatory genes yclJK upon oxygen limitation
- PMID: 15375128
- PMCID: PMC516614
- DOI: 10.1128/JB.186.19.6477-6484.2004
Bacillus subtilis ResD induces expression of the potential regulatory genes yclJK upon oxygen limitation
Abstract
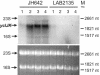
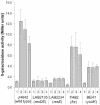
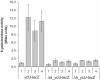
Transcription of the yclJK operon, which encodes a potential two-component regulatory system, is activated in response to oxygen limitation in Bacillus subtilis. Northern blot analysis and assays of yclJ-lacZ reporter gene fusion activity revealed that the anaerobic induction is dependent on another two-component signal transduction system encoded by resDE. ResDE was previously shown to be required for the induction of anaerobic energy metabolism. Electrophoretic mobility shift assays and DNase I footprinting experiments showed that the response regulator ResD binds specifically to the yclJK regulatory region upstream of the transcriptional start site. In vitro transcription experiments demonstrated that ResD is sufficient to activate yclJ transcription. The phosphorylation of ResD by its sensor kinase, ResE, highly stimulates its activity as a transcriptional activator. Multiple nucleotide substitutions in the ResD binding regions of the yclJ promoter abolished ResD binding in vitro and prevented the anaerobic induction of yclJK in vivo. A weight matrix for the ResD binding site was defined by a bioinformatic approach. The results obtained suggest the existence of a new branch of the complex regulatory system employed for the adaptation of B. subtilis to anaerobic growth conditions.
Figures

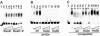



Similar articles
-
The Bacillus subtilis nrdEF genes, encoding a class Ib ribonucleotide reductase, are essential for aerobic and anaerobic growth.Appl Environ Microbiol. 2006 Aug;72(8):5260-5. doi: 10.1128/AEM.00599-06. Appl Environ Microbiol. 2006. PMID: 16885274 Free PMC article.
-
Characterization of ResDE-dependent fnr transcription in Bacillus subtilis.J Bacteriol. 2007 Mar;189(5):1745-55. doi: 10.1128/JB.01502-06. Epub 2006 Dec 22. J Bacteriol. 2007. PMID: 17189364 Free PMC article.
-
Regulation of respiratory genes by ResD-ResE signal transduction system in Bacillus subtilis.Methods Enzymol. 2007;422:448-64. doi: 10.1016/S0076-6879(06)22023-8. Methods Enzymol. 2007. PMID: 17628154
-
Regulation of the anaerobic metabolism in Bacillus subtilis.Adv Microb Physiol. 2012;61:195-216. doi: 10.1016/B978-0-12-394423-8.00005-6. Adv Microb Physiol. 2012. PMID: 23046954 Review.
-
Anaerobic growth of a "strict aerobe" (Bacillus subtilis).Annu Rev Microbiol. 1998;52:165-90. doi: 10.1146/annurev.micro.52.1.165. Annu Rev Microbiol. 1998. PMID: 9891797 Review.
Cited by
-
The blue light-dependent LOV-protein LdaP of Dinoroseobacter shibae acts as antirepressor of the PpsR repressor, regulating photosynthetic gene cluster expression.Front Microbiol. 2024 Feb 7;15:1351297. doi: 10.3389/fmicb.2024.1351297. eCollection 2024. Front Microbiol. 2024. PMID: 38404597 Free PMC article.
-
Bacillus subtilis YngB contributes to wall teichoic acid glucosylation and glycolipid formation during anaerobic growth.J Biol Chem. 2021 Jan-Jun;296:100384. doi: 10.1016/j.jbc.2021.100384. Epub 2021 Feb 5. J Biol Chem. 2021. PMID: 33556370 Free PMC article.
-
The Fnr regulon of Bacillus subtilis.J Bacteriol. 2006 Feb;188(3):1103-12. doi: 10.1128/JB.188.3.1103-1112.2006. J Bacteriol. 2006. PMID: 16428414 Free PMC article.
-
Identification of regulatory RNAs in Bacillus subtilis.Nucleic Acids Res. 2010 Oct;38(19):6637-51. doi: 10.1093/nar/gkq454. Epub 2010 Jun 4. Nucleic Acids Res. 2010. PMID: 20525796 Free PMC article.
-
Spatio-temporal remodeling of functional membrane microdomains organizes the signaling networks of a bacterium.PLoS Genet. 2015 Apr 24;11(4):e1005140. doi: 10.1371/journal.pgen.1005140. eCollection 2015 Apr. PLoS Genet. 2015. PMID: 25909364 Free PMC article.
References
-
- Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.). 1995. Current protocols in molecular biology. John Wiley & Sons, Inc., New York, N.Y.
-
- Engler-Blum, G., M. Meier, J. Frank, and G. A. Müller. 1993. Reduction of background problems in nonradioactive Northern and Southern blot analyses enables higher sensitivity than 32P-based hybridizations. Anal. Biochem. 210:235-244. - PubMed
-
- Erlendsson, L. S., R. M. Acheson, L. Hederstedt, and N. E. Le Brun. 2003. Bacillus subtilis ResA is a thiol-disulfide oxidoreductase involved in cytochrome c synthesis. J. Biol. Chem. 278:17852-17858. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

