A multifunction ABC transporter (Opt) contributes to diversity of peptide uptake specificity within the genus Lactococcus
- PMID: 15375130
- PMCID: PMC516603
- DOI: 10.1128/JB.186.19.6492-6500.2004
A multifunction ABC transporter (Opt) contributes to diversity of peptide uptake specificity within the genus Lactococcus
Abstract
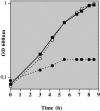
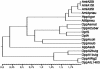
Growth of Lactococcus lactis in milk depends on the utilization of extracellular peptides. Up to now, oligopeptide uptake was thought to be due only to the ABC transporter Opp. Nevertheless, analysis of several Opp-deficient L. lactis strains revealed the implication of a second oligopeptide ABC transporter, the so-called Opt system. Both transporters are expressed in wild-type strains such as L. lactis SK11 and Wg2, whereas the plasmid-free strains MG1363 and IL-1403 synthesize only Opp and Opt, respectively. The Opt system displays significant differences from the lactococcal Opp system, which made Opt much more closely related to the oligopeptide transporters of streptococci than to the lactococcal Opp system: (i) genetic organization, (ii) peptide uptake specificity, and (iii) presence of two oligopeptide-binding proteins, OptS and OptA. The fact that only OptA is required for nutrition calls into question the function of the second oligopeptide binding protein (Opts). Sequence analysis of oligopeptide-binding proteins from different bacteria prompted us to propose a classification of these proteins in three distinct groups, differentiated by the presence (or not) of precisely located extensions.
Figures



Similar articles
-
The peptide transport system Opt is involved in both nutrition and environmental sensing during growth of Lactococcus lactis in milk.Microbiology (Reading). 2011 Jun;157(Pt 6):1612-1619. doi: 10.1099/mic.0.048173-0. Epub 2011 Mar 10. Microbiology (Reading). 2011. PMID: 21393368
-
Diversity of oligopeptide transport specificity in Lactococcus lactis species. A tool to unravel the role of OppA in uptake specificity.J Biol Chem. 2003 Apr 25;278(17):14832-40. doi: 10.1074/jbc.M212454200. Epub 2003 Feb 16. J Biol Chem. 2003. PMID: 12590143
-
Genetic and biochemical characterization of the oligopeptide transport system of Lactococcus lactis.J Bacteriol. 1993 Dec;175(23):7523-32. doi: 10.1128/jb.175.23.7523-7532.1993. J Bacteriol. 1993. PMID: 8244921 Free PMC article.
-
Specificity and selectivity determinants of peptide transport in Lactococcus lactis and other microorganisms.Mol Microbiol. 2005 Aug;57(3):640-9. doi: 10.1111/j.1365-2958.2005.04698.x. Mol Microbiol. 2005. PMID: 16045610 Review.
-
Multidrug transporters and antibiotic resistance in Lactococcus lactis.Biochim Biophys Acta. 2002 Sep 10;1555(1-3):1-7. doi: 10.1016/s0005-2728(02)00246-3. Biochim Biophys Acta. 2002. PMID: 12206883 Review.
Cited by
-
Defining the Environmental Adaptations of Genus Devosia: Insights into its Expansive Short Peptide Transport System and Positively Selected Genes.Sci Rep. 2020 Jan 24;10(1):1151. doi: 10.1038/s41598-020-58163-8. Sci Rep. 2020. PMID: 31980727 Free PMC article.
-
Investigation of the adaptation of Lactococcus lactis to isoleucine starvation integrating dynamic transcriptome and proteome information.Microb Cell Fact. 2011 Aug 30;10 Suppl 1(Suppl 1):S18. doi: 10.1186/1475-2859-10-S1-S18. Epub 2011 Aug 30. Microb Cell Fact. 2011. PMID: 21995707 Free PMC article.
-
Only one of four oligopeptide transport systems mediates nitrogen nutrition in Staphylococcus aureus.J Bacteriol. 2007 Jul;189(14):5119-29. doi: 10.1128/JB.00274-07. Epub 2007 May 11. J Bacteriol. 2007. PMID: 17496096 Free PMC article.
-
Proline betaine uptake in Sinorhizobium meliloti: Characterization of Prb, an opp-like ABC transporter regulated by both proline betaine and salinity stress.J Bacteriol. 2006 Sep;188(17):6308-17. doi: 10.1128/JB.00585-06. J Bacteriol. 2006. PMID: 16923898 Free PMC article.
-
Streptococcus pneumoniae Proteins AmiA, AliA, and AliB Bind Peptides Found in Ribosomal Proteins of Other Bacterial Species.Front Microbiol. 2018 Jan 15;8:2688. doi: 10.3389/fmicb.2017.02688. eCollection 2017. Front Microbiol. 2018. PMID: 29379482 Free PMC article.
References
-
- Ajdic, D., W. M. McShan, R. E. McLaughlin, G. Savic, J. Chang, M. B. Carson, C. Primeaux, R. Tian, S. Kenton, H. Jia, S. Lin, Y. Qian, S. Li, H. Zhu, F. Najar, H. Lai, J. White, B. A. Roe, and J. Ferretti. 2002. Genome sequence of Streptococcus mutans UA159, a cariogenic dental pathogen. Proc. Natl. Acad. Sci. USA 99:14434-14439. - PMC - PubMed
-
- Alloing, G., P. de Philip, and J.-P. Claverys. 1994. Three highly homologous membrane-bound lipoproteins participate in oligopeptide transport by the Ami system of the gram-positive Streptococcus pneumoniae. J. Mol. Biol. 241:44-58. - PubMed
-
- Aubel, D., J. E. Germond, C. Gilbert, and D. Atlan. 2002. Isolation of the patC gene encoding the cystathionine beta-lyase of Lactobacillus delbrueckii subsp. bulgaricus and molecular analysis of inter-strain variability in enzyme biosynthesis. Microbiology 148:2029-2036. - PubMed
-
- Charbonnel, P., M. Lamarque, J.-C. Piard, C. Gilbert, V. Juillard, and D. Atlan. 2003. Diversity of oligopeptide transport specificity in Lactococcus lactis species: a tool to unravel the role of OppA in uptake specificity. J. Biol. Chem. 278:14832-14840. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

