Importance of Rhodospirillum rubrum H(+)-pyrophosphatase under low-energy conditions
- PMID: 15375148
- PMCID: PMC516592
- DOI: 10.1128/JB.186.19.6651-6655.2004
Importance of Rhodospirillum rubrum H(+)-pyrophosphatase under low-energy conditions
Abstract
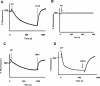
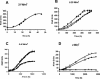
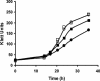
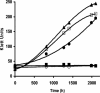
The physiological role of the membrane-bound pyrophosphatase of Rhodospirillum rubrum was investigated by the characterization of a mutant strain. Comparisons of growth levels between the wild type and the mutant under different low-potential conditions and during transitions between different metabolisms indicate that this enzyme provides R. rubrum with an alternative energy source that is important for growth in low-energy states.
Figures
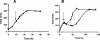
Similar articles
-
Differential regulation of soluble and membrane-bound inorganic pyrophosphatases in the photosynthetic bacterium Rhodospirillum rubrum provides insights into pyrophosphate-based stress bioenergetics.J Bacteriol. 2004 Aug;186(16):5418-26. doi: 10.1128/JB.186.16.5418-5426.2004. J Bacteriol. 2004. PMID: 15292143 Free PMC article.
-
H+/PPi stoichiometry of membrane-bound pyrophosphatase of Rhodospirillum rubrum.Arch Biochem Biophys. 1995 Jan 10;316(1):421-7. doi: 10.1006/abbi.1995.1056. Arch Biochem Biophys. 1995. PMID: 7840646
-
Energy-linked reactions in photosynthetic bacteria: Pi in equilibrium with HOH oxygen exchange catalyzed by the membrane-bound inorganic pyrophosphatase of Rhodospirillum rubrum.Arch Biochem Biophys. 1981 May;208(2):426-30. doi: 10.1016/0003-9861(81)90528-2. Arch Biochem Biophys. 1981. PMID: 6114711 No abstract available.
-
H+-PPases: yesterday, today and tomorrow.IUBMB Life. 2007 Feb;59(2):76-83. doi: 10.1080/15216540701258132. IUBMB Life. 2007. PMID: 17454298 Review.
-
Studies on the light-dependent synthesis of inorganic pyrophosphate by Rhodospirillum rubrum chromatophores.Biochem J. 1972 Sep;129(2):571-81. Biochem J. 1972. PMID: 4345276 Free PMC article.
Cited by
-
Expression patterns reveal niche diversification in a marine microbial assemblage.ISME J. 2013 Feb;7(2):281-98. doi: 10.1038/ismej.2012.96. Epub 2012 Aug 30. ISME J. 2013. PMID: 22931830 Free PMC article.
-
Functional and structural asymmetry suggest a unifying principle for catalysis in membrane-bound pyrophosphatases.EMBO Rep. 2024 Feb;25(2):853-875. doi: 10.1038/s44319-023-00037-x. Epub 2024 Jan 5. EMBO Rep. 2024. PMID: 38182815 Free PMC article.
-
Pyrophosphate-fueled Na+ and H+ transport in prokaryotes.Microbiol Mol Biol Rev. 2013 Jun;77(2):267-76. doi: 10.1128/MMBR.00003-13. Microbiol Mol Biol Rev. 2013. PMID: 23699258 Free PMC article. Review.
-
Pre-steady-state kinetics and solvent isotope effects support the "billiard-type" transport mechanism in Na+ -translocating pyrophosphatase.Protein Sci. 2022 Sep;31(9):e4394. doi: 10.1002/pro.4394. Protein Sci. 2022. PMID: 36040263 Free PMC article.
-
Method for the facile transformation of marine purple photosynthetic bacteria using chemically competent cells.Microbiologyopen. 2020 Jan;9(1):e00953. doi: 10.1002/mbo3.953. Epub 2019 Oct 22. Microbiologyopen. 2020. PMID: 31638342 Free PMC article.
References
-
- Baltscheffsky, H., L. V. von Stedingk, H. W. Heldt, and M. Klingenberg. 1966. Inorganic pyrophosphate: formation in bacterial photophosphorylation. Science 153:1120-1122. - PubMed
-
- Baykov, A. A., N. V. Sergina, O. A. Evtushenko, and E. B. Dubnova. 1996. Kinetic characterization of the hydrolytic activity of the H+ pyrophosphatase of Rhodospirillum rubrum in membrane-bound and isolated states. Eur. J. Biochem. 236:121-127. - PubMed
-
- Celis, H., and I. Romero. 1987. The phosphate-pyrophosphate exchange and hydrolytic reactions of the membrane-bound pyrophosphatase of Rhodospirillum rubrum: effects of pH and divalent cations. J. Bioenerg. Biomembr. 19:255-272. - PubMed
-
- Celis, H., B. Franco, S. Escobedo, and I. Romero. 2003. Rhodobacter sphaeroides has a family II pyrophosphatase. Comparison with other species of photosynthetic bacteria. Arch. Microbiol. 179:368-376. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

