Channel-forming (Porin) activity in Herpetosiphon aurantiacus Hp a2
- PMID: 15375151
- PMCID: PMC516602
- DOI: 10.1128/JB.186.19.6667-6670.2004
Channel-forming (Porin) activity in Herpetosiphon aurantiacus Hp a2
Abstract
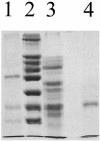
Detergent extracts of cell envelopes of the gliding bacterium Herpetosiphon aurantiacus formed channels in lipid bilayers. Fast protein liquid chromatography across a HiTrap-Q cation-exchange column demonstrated that a 45-kDa protein forms the channel. The observation of a channel-forming protein suggests that Herpetosiphon aurantiacus Hp a2 has a permeability barrier on its surface.
Figures


References
-
- Behlau, M., D. J. Mills, H. Quader, W. Kühlbrand, and J. Vonck. 2001. Projection structure of the monomeric porin OmpG at 6 Å resolution. J. Mol. Biol. 305:71-77. - PubMed
-
- Benz, R. 2001. Porins—structure and function, p. 227-246. In G. Winkelmann (ed.), Microbial transport systems. Wiley-VCH Verlag GmbH, Weinheim, Germany.
-
- Benz, R., K. Janko, W. Boos, and A. Läuger. 1978. Formation of large, ion-permeable membrane channels by the matrix protein (porin) of Escherichia coli. Biochim. Biophys. Acta 511:305-319. - PubMed
-
- Benz, R., K. Janko, and P. Läuger. 1979. Ionic selectivity of pores formed by the matrix protein (porin) of Escherichia coli. Biochim. Biophys. Acta 551:238-247. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

