Safety assessment of inhaled xylitol in mice and healthy volunteers
- PMID: 15377394
- PMCID: PMC521689
- DOI: 10.1186/1465-9921-5-13
Safety assessment of inhaled xylitol in mice and healthy volunteers
Abstract
Background: Xylitol is a 5-carbon sugar that can lower the airway surface salt concentration, thus enhancing innate immunity. We tested the safety and tolerability of aerosolized iso-osmotic xylitol in mice and human volunteers.
Methods: This was a prospective cohort study of C57Bl/6 mice in an animal laboratory and healthy human volunteers at the clinical research center of a university hospital. Mice underwent a baseline methacholine challenge, exposure to either aerosolized saline or xylitol (5% solution) for 150 minutes and then a follow-up methacholine challenge. The saline and xylitol exposures were repeated after eosinophilic airway inflammation was induced by sensitization and inhalational challenge to ovalbumin. Normal human volunteers underwent exposures to aerosolized saline (10 ml) and xylitol, with spirometry performed at baseline and after inhalation of 1, 5, and 10 ml. Serum osmolarity and electrolytes were measured at baseline and after the last exposure. A respiratory symptom questionnaire was administered at baseline, after the last exposure, and five days after exposure. In another group of normal volunteers, bronchoalveolar lavage (BAL) was done 20 minutes and 3 hours after aerosolized xylitol exposure for levels of inflammatory markers.
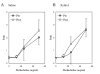
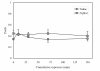
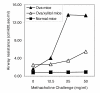
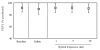
Results: In naive mice, methacholine responsiveness was unchanged after exposures to xylitol compared to inhaled saline (p = 0.49). There was no significant increase in Penh in antigen-challenged mice after xylitol exposure (p = 0.38). There was no change in airway cellular response after xylitol exposure in naive and antigen-challenged mice. In normal volunteers, there was no change in FEV1 after xylitol exposures compared with baseline as well as normal saline exposure (p = 0.19). Safety laboratory values were also unchanged. The only adverse effect reported was stuffy nose by half of the subjects during the 10 ml xylitol exposure, which promptly resolved after exposure completion. BAL cytokine levels were below the detection limits after xylitol exposure in normal volunteers.
Conclusions: Inhalation of aerosolized iso-osmotic xylitol was well-tolerated by naive and atopic mice, and by healthy human volunteers.
Figures
References
-
- Huttner KM, Bevins CL. Antimicrobial peptides as mediators of epithelial host defense. Pediatr Res. 1999;45:785–794. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

