The plant-specific cyclin-dependent kinase CDKB1;1 and transcription factor E2Fa-DPa control the balance of mitotically dividing and endoreduplicating cells in Arabidopsis
- PMID: 15377755
- PMCID: PMC520964
- DOI: 10.1105/tpc.104.024398
The plant-specific cyclin-dependent kinase CDKB1;1 and transcription factor E2Fa-DPa control the balance of mitotically dividing and endoreduplicating cells in Arabidopsis
Abstract
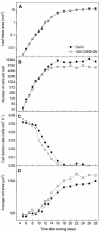
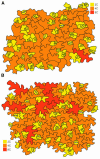
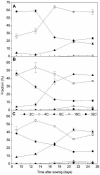
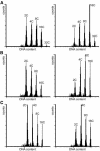
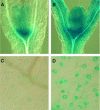
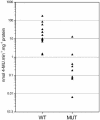
Transgenic Arabidopsis thaliana plants overproducing the E2Fa-DPa transcription factor have two distinct cell-specific phenotypes: some cells divide ectopically and others are stimulated to endocycle. The decision of cells to undergo extra mitotic divisions has been postulated to depend on the presence of a mitosis-inducing factor (MIF). Plants possess a unique class of cyclin-dependent kinases (CDKs; B-type) for which no ortholog is found in other kingdoms. The peak of CDKB1;1 activity around the G2-M boundary suggested that it might be part of the MIF. Plants that overexpressed a dominant negative allele of CDKB1;1 underwent enhanced endoreduplication, demonstrating that CDKB1;1 activity was required to inhibit the endocycle. Moreover, when the mutant CDKB1;1 allele was overexpressed in an E2Fa-DPa-overproducing background, it enhanced the endoreduplication phenotype, whereas the extra mitotic cell divisions normally induced by E2Fa-DPa were repressed. Surprisingly, CDKB1;1 transcription was controlled by the E2F pathway, as shown by its upregulation in E2Fa-DPa-overproducing plants and mutational analysis of the E2F binding site in the CDKB1;1 promoter. These findings illustrate a cross talking mechanism between the G1-S and G2-M transition points.
Figures


References
-
- Brodsky, V.Y., and Uryvaeva, I.V. (1977). Cell polyploidy: Its relation to tissue growth and function. Int. Rev. Cytol. 50, 275–332. - PubMed
-
- D'Amato, F. (1964). Endopolyploidy as a factor in plant tissue development. Caryologia 17, 41–52.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

