A classical arabinogalactan protein is essential for the initiation of female gametogenesis in Arabidopsis
- PMID: 15377758
- PMCID: PMC520959
- DOI: 10.1105/tpc.104.024588
A classical arabinogalactan protein is essential for the initiation of female gametogenesis in Arabidopsis
Abstract
Classical arabinogalactan proteins (AGPs) are an abundant class of cell surface proteoglycans widely distributed in flowering plants. We have used a combination of enhancer detection tagging and RNA interference (RNAi)-induced posttrancriptional silencing to demonstrate that AGP18, a gene encoding a classical arabinogalactan protein, is essential for female gametogenesis in Arabidopsis thaliana. AGP18 is expressed in cells that spatially and temporally define the sporophytic to gametophytic transition and during early stages of seed development. More than 75% of the T1 transformants resulted in T2 lines showing reduced seed set during at least three consecutive generations but no additional developmental defects. AGP18-silenced T2 lines showed reduced AGP18 transcript levels in female reproductive organs, the presence of 21-bp RNA fragments specific to the AGP18 gene, and the absence of in situ AGP18 mRNA localization in developing ovules. Reciprocal crosses to wild-type plants indicate that the defect is female specific. The genetic and molecular analysis of AGP18-silenced plants containing a single T-DNA RNAi insertion suggests that posttranscriptional silencing of AGP18 is acting both at the sporophytic and gametophytic levels. A cytological analysis of all defective AGP18-RNAi lines, combined with the analysis of molecular markers acting at key stages of female gametogenesis, showed that the functional megaspore fails to enlarge and mitotically divide, indicating that AGP18 is essential to initiate female gametogenesis in Arabidopsis. Our results assign a specific function in plant development to a gene encoding a classical AGP.
Figures

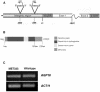


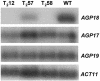



References
-
- Bajon, C., Horlow, C., Motamayor, J.C., Sauvanet, A., and Robert, D. (1999). Megasporogenesis in Arabidopsis thaliana L.: An ultrastructural study. Sex. Plant Reprod. 12, 99–109.
-
- Bell, P.R. (1989). The alternation of generations. Adv. Bot. Res. 16, 55–93.
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

