Biosynthesis of very-long-chain polyunsaturated fatty acids in transgenic oilseeds: constraints on their accumulation
- PMID: 15377762
- PMCID: PMC520968
- DOI: 10.1105/tpc.104.026070
Biosynthesis of very-long-chain polyunsaturated fatty acids in transgenic oilseeds: constraints on their accumulation
Abstract
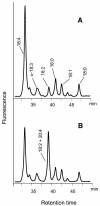
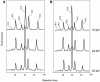
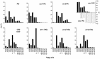
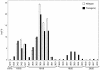
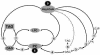
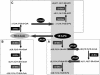
Omega6- and omega3-polyunsaturated C20 fatty acids represent important components of the human diet. A more regular consumption and an accordingly sustainable source of these compounds are highly desirable. In contrast with the very high levels to which industrial fatty acids have to be enriched in plant oils for competitive use as chemical feedstocks, much lower percentages of very-long-chain polyunsaturated fatty acids (VLCPUFA) in edible plant oils would satisfy nutritional requirements. Seed-specific expression in transgenic tobacco (Nicotiana tabacum) and linseed (Linum usitatissimum) of cDNAs encoding fatty acyl-desaturases and elongases, absent from all agronomically important plants, resulted in the very high accumulation of Delta6-desaturated C18 fatty acids and up to 5% of C20 polyunsaturated fatty acids, including arachidonic and eicosapentaenoic acid. Detailed lipid analyses of developing seeds from transgenic plants were interpretated as indicating that, after desaturation on phosphatidylcholine, Delta6-desaturated products are immediately channeled to the triacylglycerols and effectively bypass the acyl-CoA pool. Thus, the lack of available Delta6-desaturated acyl-CoA substrates in the acyl-CoA pool limits the synthesis of elongated C20 fatty acids and disrupts the alternating sequence of lipid-linked desaturations and acyl-CoA dependent elongations. As well as the successful production of VLCPUFA in transgenic oilseeds and the identification of constraints on their accumulation, our results indicate alternative strategies to circumvent this bottleneck.
Figures


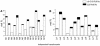

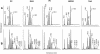
References
-
- Abbadi, A., Domergue, F., Meyer, A., Riedel, K., Sperling, P., Zank, T., and Heinz, E. (2001). Transgenic oilseeds as sustainable sources of nutritionally relevant C20 and C22 polyunsaturated fatty acids? Eur. J. Lipid Sci. Technol. 103, 106–113.
-
- Agius, F., Gonzalez-Lamothe, R., Caballero, J.L., Munoz-Blanco, J., Botella, M.A., and Valpuesta, V. (2003). Engineering increased vitamin C levels in plants by overexpression of a D-galacturonic acid reductase. Nat. Biotechnol 21, 177–181. - PubMed
-
- Bäumlein, H., Boerjan, W., Nagy, I., Bassuner, R., Van Montagu, M., Inze, D., and Wobus, U. (1991). A novel seed protein gene from Vicia faba is developmentally regulated in transgenic tobacco and Arabidopsis plants. Mol. Gen. Genet. 225, 459–467. - PubMed
-
- Beaudoin, F., and Napier, J.A. (2004). Biosynthesis and compartmentation of triacylglycerol in higher plants. In Lipid Metabolism and Membrane Biogenesis, G. Daum, ed (Berlin: Springer), pp. 267–287.
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

