Regulation of sulfate uptake and expression of sulfate transporter genes in Brassica oleracea as affected by atmospheric H(2)S and pedospheric sulfate nutrition
- PMID: 15377780
- PMCID: PMC523398
- DOI: 10.1104/pp.104.046441
Regulation of sulfate uptake and expression of sulfate transporter genes in Brassica oleracea as affected by atmospheric H(2)S and pedospheric sulfate nutrition
Abstract
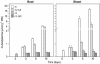
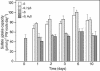
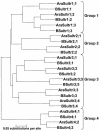
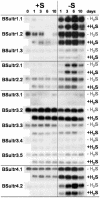
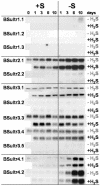
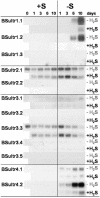
Demand-driven signaling will contribute to regulation of sulfur acquisition and distribution within the plant. To investigate the regulatory mechanisms pedospheric sulfate and atmospheric H(2)S supply were manipulated in Brassica oleracea. Sulfate deprivation of B. oleracea seedlings induced a rapid increase of the sulfate uptake capacity by the roots, accompanied by an increased expression of genes encoding specific sulfate transporters in roots and other plant parts. More prolonged sulfate deprivation resulted in an altered shoot-root partitioning of biomass in favor of the root. B. oleracea was able to utilize atmospheric H(2)S as S-source; however, root proliferation and increased sulfate transporter expression occurred as in S-deficient plants. It was evident that in B. oleracea there was a poor shoot to root signaling for the regulation of sulfate uptake and expression of the sulfate transporters. cDNAs corresponding to 12 different sulfate transporter genes representing the complete gene family were isolated from Brassica napus and B. oleracea species. The sequence analysis classified the Brassica sulfate transporter genes into four different groups. The expression of the different sulfate transporters showed a complex pattern of tissue specificity and regulation by sulfur nutritional status. The sulfate transporter genes of Groups 1, 2, and 4 were induced or up-regulated under sulfate deprivation, although the expression of Group 3 sulfate transporters was not affected by the sulfate status. The significance of sulfate, thiols, and O-acetylserine as possible signal compounds in the regulation of the sulfate uptake and expression of the transporter genes is evaluated.
Figures

References
-
- Castro A, Stulen I, De Kok LJ (2003) Nitrogen and sulfur requirement of Brassica oleracea L. cultivars. In J-C Davidian, D Grill, LJ de Kok, I Stulen, MJ Hawkesford, E Schnug, H Rennenberg, eds, Sulfur Transport and Assimilation in Plants. Backhuys Publishers, Leiden, The Netherlands, pp 181–183
-
- Clarkson DT, Saker LR, Purves JV (1989) Depression of nitrate and ammonium transport in barley plants with diminished sulphate status. Evidence for co-regulation of nitrogen and sulphate uptake. J Exp Bot 40: 953–963
-
- Clarkson DT, Hawkesford MJ, Davidian J-C (1993) Membrane and long-distance transport of sulfate. In LJ De Kok, I Stulen, H Rennenberg, C Brunold, WE Rauser, eds, Sulfur Nutrition and Sulfur Assimilation in Higher Plants: Fundamental, Environmental and Agricultural Aspects. SPB Academic Publishing, The Hague, pp 3–19
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

