Haemophilus influenzae type b conjugate vaccines
- PMID: 15379976
- PMCID: PMC1782565
- DOI: 10.1111/j.1365-2567.2004.01971.x
Haemophilus influenzae type b conjugate vaccines
Abstract
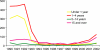
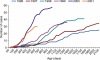
Haemophilus influenzae type b (Hib) is one of the leading causes of invasive bacterial infection in young children worldwide. During childhood, acquisition of antibody directed against the polysaccharide capsule of the organism, presumably as a result of asymptomatic carriage, confers protection and disease is much less common after the age of 4 years. Like other polysaccharides, the polyribosyl ribitol phosphate (PRP) of the Hib capsule is a T-independent antigen and not immunogenic when administered as a vaccine in infancy. Because the highest rates of disease occur in the first 2 years of life, efficacious Hib vaccines have been designed by covalently linking the PRP capsule to a carrier protein that recruits T-cell help for the polysaccharide immune response and induces anti-PRP antibody production even in the first 6 months of life. Introduction of Hib protein-polysaccharide conjugate vaccines into many industrialized countries over the past 15 years has resulted in the virtual elimination of invasive Hib disease. However, despite the success of the vaccine programme several factors may interfere with the effectiveness of the vaccine in the routine programme, as observed in the UK recently. Such factors may include interference with other concomitant vaccines, waning immunity in the absence of booster doses of vaccine, and reduced natural boosting as a result of decreased transmission of the organism. However, the burden of disease remains highest in resource-poor countries and urgent efforts are needed to provide the benefits of this vaccine for children living in regions where it cannot be used for economic and logistical reasons.
Figures
References
-
- Weller PF, Smith AL, Smith DH, Anderson P. Role of immunity in the clearance of bacteremia due to Haemophilus influenzae. J Infect Dis. 1978;138:427. - PubMed
-
- Moxon ER, Kroll JS. The role of bacterial polysaccharide capsules as virulence factors. Curr Top Microbiol Immunol. 1990;150:65. - PubMed
-
- Swift AJ, Moxon ER, Zwahlen A, Winkelstein JA. Complement-mediated serum activities against genetically defined capsular transformants of Haemophilus influenzae. Microb Pathog. 1991;10:261. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
