Increased circulating levels and salivary gland expression of interleukin-18 in patients with Sjögren's syndrome: relationship with autoantibody production and lymphoid organization of the periductal inflammatory infiltrate
- PMID: 15380044
- PMCID: PMC546280
- DOI: 10.1186/ar1209
Increased circulating levels and salivary gland expression of interleukin-18 in patients with Sjögren's syndrome: relationship with autoantibody production and lymphoid organization of the periductal inflammatory infiltrate
Abstract
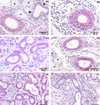
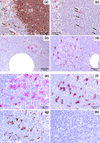
IL-18, an immunoregulatory and proinflammatory cytokine, has been shown to play an important pathogenic role in Th1-driven autoimmune disorders. In this study, we evaluated the circulating levels and salivary-gland expression of IL-18 in patients with Sjögren's syndrome (SS), a mainly Th1-mediated disease. IL-18 serum levels were measured by ELISA in 37 patients with primary SS, 42 with rheumatoid arthritis, and 21 normal controls. We demonstrated high IL-18 serum levels in SS, similar to those in rheumatoid arthritis patients and significantly higher than in controls (P < 0.01). In addition, IL-18 serum concentrations were significantly higher in anti-SSA/Ro+ and anti-SSB/La+ than in anti-SSA/Ro- and anti-SSB/La- SS patients (respectively, P = 0.01, P < 0.01). Serum IL-18 correlated strongly with anti-SSA/Ro (P = 0.004) and anti-SSB/La (P = 0.01) titers. Salivary gland IL-18 expression was investigated by single/double immunohistochemistry in 13 patients with primary SS and in 10 with chronic sialoadenitis, used as controls. The expression of IL-18 was also examined in periductal inflammatory foci in relation to the acquisition of features of secondary lymphoid organs such as T-B compartmentalization, formation of follicular dendritic cell networks, and presence of germinal-center-like structures. IL-18 expression in SS salivary glands was detected in 28 of 32 periductal foci of mononuclear cells (87.5%), while no IL-18 production by infiltrating cells was detected in patients with chronic sialoadenitis. Within the inflammatory foci, IL-18 immunoreactivity co-localized almost exclusively with CD68+ macrophages. In addition, IL-18 was found in 15 of 19 foci (78.9%) with no evidence of T-B cell compartmentalization (nonsegregated) but in 100% of the segregated aggregates, both in T- and B-cell-rich areas. Strikingly, IL-18 was strongly expressed by CD68+ tingible body macrophages in germinal-centre-like structures both in SS salivary glands and in normal lymph nodes. IL-18 expression was observed in the ducts of all SS biopsies but in only 4 of 10 patients with nonspecific chronic sialoadenitis (P < 0.01). This study provides the first evidence of increased circulating levels and salivary gland expression of IL-18 in SS, suggesting an important contribution of this cytokine to the modulation of immune inflammatory pathways in this condition.
Figures



Similar articles
-
Detection of anti-Ro/SSA and anti-La/SSB autoantibody-producing cells in salivary glands from patients with Sjögren's syndrome.Arthritis Rheum. 1998 Dec;41(12):2238-48. doi: 10.1002/1529-0131(199812)41:12<2238::AID-ART20>3.0.CO;2-V. Arthritis Rheum. 1998. PMID: 9870881
-
Endoplasmic reticulum stress causes autophagy and apoptosis leading to cellular redistribution of the autoantigens Ro/Sjögren's syndrome-related antigen A (SSA) and La/SSB in salivary gland epithelial cells.Clin Exp Immunol. 2015 Aug;181(2):244-52. doi: 10.1111/cei.12638. Clin Exp Immunol. 2015. PMID: 25845745 Free PMC article.
-
The association between autoantibody types and salivary gland hypofunction in patients with primary Sjögren's syndrome.J Oral Pathol Med. 2023 Feb;52(2):188-194. doi: 10.1111/jop.13369. Epub 2022 Nov 2. J Oral Pathol Med. 2023. PMID: 36183157
-
Modes and Mechanisms of Salivary Gland Epithelial Cell Death in Sjogren's Syndrome.Adv Biol (Weinh). 2023 Dec;7(12):e2300173. doi: 10.1002/adbi.202300173. Epub 2023 Jul 6. Adv Biol (Weinh). 2023. PMID: 37409392 Review.
-
Sjögren's syndome and extragonadal sex steroid formation: a clue to a better disease control?J Steroid Biochem Mol Biol. 2015 Jan;145:237-44. doi: 10.1016/j.jsbmb.2014.08.014. Epub 2014 Aug 23. J Steroid Biochem Mol Biol. 2015. PMID: 25158020 Review.
Cited by
-
Current Aspects of Pathogenesis in Sjögren's Syndrome.Ther Adv Musculoskelet Dis. 2010 Dec;2(6):325-34. doi: 10.1177/1759720X10381431. Ther Adv Musculoskelet Dis. 2010. PMID: 22870458 Free PMC article.
-
Smoking, disease characteristics and serum cytokine levels in patients with primary Sjögren's syndrome.Rheumatol Int. 2018 Aug;38(8):1503-1510. doi: 10.1007/s00296-018-4063-8. Epub 2018 May 30. Rheumatol Int. 2018. PMID: 29846789 Free PMC article.
-
Myd88 is required for disease development in a primary Sjögren's syndrome mouse model.J Leukoc Biol. 2017 Dec;102(6):1411-1420. doi: 10.1189/jlb.3A0717-311R. Epub 2017 Sep 26. J Leukoc Biol. 2017. PMID: 28951424 Free PMC article.
-
Update on Pathogenesis of Sjogren's Syndrome.Curr Rheumatol Rev. 2017;13(1):5-22. doi: 10.2174/1573397112666160714164149. Curr Rheumatol Rev. 2017. PMID: 27412602 Free PMC article. Review.
-
Epistasis with HLA DR3 implicates the P2X7 receptor in the pathogenesis of primary Sjögren's syndrome.Arthritis Res Ther. 2013 Jun 2;15(4):R71. doi: 10.1186/ar4248. Arthritis Res Ther. 2013. PMID: 23819992 Free PMC article.
References
-
- Fox RI, Kang HI, Ando D, Abrams J, Pisa E. Cytokine mRNA expression in salivary gland biopsies of Sjogren's syndrome. J Immunol. 1994;152:5532–5539. - PubMed
-
- Mitsias DI, Tzioufas AG, Veiopoulou C, Zintzaras E, Tassios IK, Kogopoulou O, Moutsopoulos HM, Thyphronitis G. The Th1/Th2 cytokine balance changes with the progress of the immunopathological lesion of Sjogren's syndrome. Clin Exp Immunol. 2002;128:562–568. doi: 10.1046/j.1365-2249.2002.01869.x. - DOI - PMC - PubMed
-
- Ohyama Y, Nakamura S, Matsuzaki G, Shinohara M, Hiroki A, Fujimura T, Yamada A, Itoh K, Nomoto K. Cytokine messenger RNA expression in the labial salivary glands of patients with Sjogren's syndrome. Arthritis Rheum. 1996;39:1376–1384. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

