Targeted disruption of the Walker-Warburg syndrome gene Pomt1 in mouse results in embryonic lethality
- PMID: 15383666
- PMCID: PMC521095
- DOI: 10.1073/pnas.0405899101
Targeted disruption of the Walker-Warburg syndrome gene Pomt1 in mouse results in embryonic lethality
Abstract
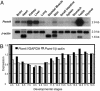
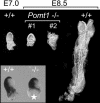
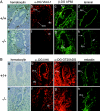
O-mannosylation is an important protein modification in eukaryotes that is initiated by an evolutionarily conserved family of protein O-mannosyltransferases. The first mammalian protein O-mannosyltransferase gene described was the human POMT1. Mutations in the hPOMT1 gene are responsible for Walker-Warburg syndrome (WWS), a severe recessive congenital muscular dystrophy associated with defects in neuronal migration that produce complex brain and eye abnormalities. During embryogenesis, the murine Pomt1 gene is prominently expressed in the neural tube, the developing eye, and the mesenchyme. These sites of expression correlate with those in which the main tissue alterations are observed in WWS patients. We have inactivated a Pomt1 allele by gene targeting in embryonic stem cells and produced chimeras transmitting the defect allele to offspring. Although heterozygous mice were viable and fertile, the total absence of Pomt1(-/-) pups in the progeny of heterozygous intercrosses indicated that this genotype is embryonic lethal. An analysis of the mutant phenotype revealed that homozygous Pomt1(-/-) mice suffer developmental arrest around embryonic day (E) 7.5 and die between E7.5 and E9.5. The Pomt1(-/-) embryos present defects in the formation of Reichert's membrane, the first basement membrane to form in the embryo. The failure of this membrane to form appears to be the result of abnormal glycosylation and maturation of dystroglycan that may impair recruitment of laminin, a structural component required for the formation of Reichert's membrane in rodents. The targeted disruption of mPomt1 represents an example of an engineered deletion of a known glycosyltransferase involved in O-mannosyl glycan synthesis.
Figures


References
-
- Strahl-Bolsinger, S., Gentzsch, M. & Tanner, W. (1999) Biochim. Biophys. Acta 1426, 297–307. - PubMed
-
- Willer, T., Valero, M. C., Tanner, W., Cruces, J. & Strahl, S. (2003) Curr. Opin. Struct. Biol. 13, 621–630. - PubMed
-
- Endo, T. & Toda, T. (2003) Biol. Pharmacol. Bull. 26, 1641–1647. - PubMed
-
- Michele, D. E. & Campbell, K. P. (2003) J. Biol. Chem. 278, 15457–15460. - PubMed
-
- Muntoni, F., Brockington, M., Torelli, S. & Brown, S. C. (2004) Curr. Opin. Neurol. 17, 205–209. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

