Insect aquaplaning: Nepenthes pitcher plants capture prey with the peristome, a fully wettable water-lubricated anisotropic surface
- PMID: 15383667
- PMCID: PMC521129
- DOI: 10.1073/pnas.0405885101
Insect aquaplaning: Nepenthes pitcher plants capture prey with the peristome, a fully wettable water-lubricated anisotropic surface
Abstract
Pitcher plants of the genus Nepenthes have highly specialized leaves adapted to attract, capture, retain, and digest arthropod prey. Several mechanisms have been proposed for the capture of insects, ranging from slippery epicuticular wax crystals to downward-pointing lunate cells and alkaloid secretions that anesthetize insects. Here we report that perhaps the most important capture mechanism has thus far remained overlooked. It is based on special surface properties of the pitcher rim (peristome) and insect "aquaplaning." The peristome is characterized by a regular microstructure with radial ridges of smooth overlapping epidermal cells, which form a series of steps toward the pitcher inside. This surface is completely wettable by nectar secreted at the inner margin of the peristome and by rain water, so that homogenous liquid films cover the surface under humid weather conditions. Only when wet, the peristome surface is slippery for insects, so that most ant visitors become trapped. By measuring friction forces of weaver ants (Oecophylla smaragdina) on the peristome surface of Nepenthes bicalcarata, we demonstrate that the two factors preventing insect attachment to the peristome, i.e., water lubrication and anisotropic surface topography, are effective against different attachment structures of the insect tarsus. Peristome water films disrupt attachment only for the soft adhesive pads but not for the claws, whereas surface topography leads to anisotropic friction only for the claws but not for the adhesive pads. Experiments on Nepenthes alata show that the trapping mechanism of the peristome is also essential in Nepenthes species with waxy inner pitcher walls.
Figures

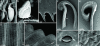

References
-
- Darwin, C. (1875) Insectivorous Plants (Appleton, London).
-
- Lloyd, F. E. (1942) The Carnivorous Plants (Ronald Press, New York).
-
- Knoll, F. (1914) Jahrb. Wiss. Bot. 54, 448–497.
-
- Adams, R. M. & Smith, G. W. (1977) Am. J. Bot. 64, 265–272.
-
- Juniper, B. E., Robins, R. J. & Joel, D. M. (1989) The Carnivorous Plants (Academic, London).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

