Systemic siRNA-mediated gene silencing: a new approach to targeted therapy of cancer
- PMID: 15383794
- PMCID: PMC1356468
- DOI: 10.1097/01.sla.0000140755.97224.9a
Systemic siRNA-mediated gene silencing: a new approach to targeted therapy of cancer
Abstract
Objective: RNA interference (RNAi), mediated by small interfering RNA (siRNA), silences genes with a high degree of specificity and potentially represents a general approach for molecularly targeted anticancer therapy. The aim of this study was to evaluate the ability of systemically administered siRNA to silence gene expression in vivo and to assess the effect of this approach on tumor growth using a murine pancreatic adenocarcinoma xenograft model.
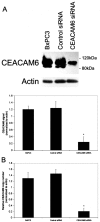
Summary background data: Carcinoembryonic antigen-related cell adhesion molecule 6 (CEACAM6) is widely overexpressed in human gastrointestinal cancer. Overexpression of CEACAM6 promotes cell survival under anchorage independent conditions, a characteristic associated with tumorigenesis and metastasis.
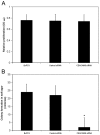
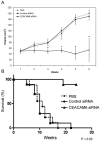
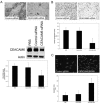
Methods: CEACAM6 expression was quantified by real-time polymerase chain reaction (PCR) and Western blot. Mice (n = 10/group) were subcutaneously xenografted with 2 x 10 BxPC3 cells (which inherently overexpress CEACAM6). Tumor growth, CEACAM6 expression, cellular proliferation (Ki-67 immunohistochemistry), apoptosis, angiogenesis (CD34 immunohistochemistry), and survival were compared for mice administered either systemic CEACAM6-specific or control single-base mismatch siRNA over 6 weeks, following orthotopic tumor implantation.
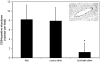
Results: Treatment with CEACAM6-specific siRNA suppressed primary tumor growth by 68% versus control siRNA (P < 0.05) and was associated with a decreased proliferating cell index, impaired angiogenesis and increased apoptosis in the xenografted tumors. CEACAM6-specific siRNA completely inhibited metastasis (0% of mice versus 60%, P < 0.05) and significantly improved survival, without apparent toxicity.
Conclusions: Our data demonstrate the efficacy of systemically administered siRNA as a therapeutic modality in experimental pancreatic cancer. This novel therapeutic strategy may be applicable to a broad range of cancers and warrants investigation in patients with refractory disease.
Figures
References
-
- Burris HA III, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–2413. - PubMed
-
- Fire A, Xu S, Montgomery MK, et al. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. - PubMed
-
- Hannon GJ. RNA interference. Nature. 2002;418:244–251. - PubMed
-
- Thompson JA, Grunert F, Zimmermann W. Carcinoembryonic antigen gene family: molecular biology and clinical perspectives. J Clin Lab Anal. 1991;5:344–366. - PubMed
-
- Kuroki M, Abe H, Imakiirei T, et al. Identification and comparison of residues critical for cell-adhesion activities of two neutrophil CD66 antigens, CEACAM6 and CEACAM8. J Leukoc Biol. 2001;70:543–550. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

