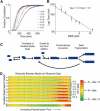
Mechanism of prion propagation: amyloid growth occurs by monomer addition
- PMID: 15383837
- PMCID: PMC517824
- DOI: 10.1371/journal.pbio.0020321
Mechanism of prion propagation: amyloid growth occurs by monomer addition
Abstract
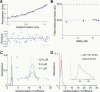
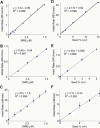
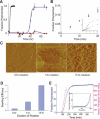
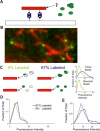
Abundant nonfibrillar oligomeric intermediates are a common feature of amyloid formation, and these oligomers, rather than the final fibers, have been suggested to be the toxic species in some amyloid diseases. Whether such oligomers are critical intermediates for fiber assembly or form in an alternate, potentially separable pathway, however, remains unclear. Here we study the polymerization of the amyloidogenic yeast prion protein Sup35. Rapid polymerization occurs in the absence of observable intermediates, and both targeted kinetic and direct single-molecule fluorescence measurements indicate that fibers grow by monomer addition. A three-step model (nucleation, monomer addition, and fiber fragmentation) accurately accounts for the distinctive kinetic features of amyloid formation, including weak concentration dependence, acceleration by agitation, and sigmoidal shape of the polymerization time course. Thus, amyloid growth can occur by monomer addition in a reaction distinct from and competitive with formation of potentially toxic oligomeric intermediates.
Conflict of interest statement
The authors have declared that no conflicts of interest exist.
Figures
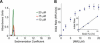
References
-
- Bradley ME, Liebman SW. The Sup35 domains required for maintenance of weak, strong or undifferentiated yeast [PSI+] prions. Mol Microbiol. 2004;51:1649–1659. - PubMed
-
- Cannon MJ, Williams AD, Wetzel R, Myszka DG. Kinetic analysis of beta-amyloid fibril elongation. Anal Biochem. 2004;328:67–75. - PubMed
-
- Caughey B, Lansbury PT. Protofibrils, pores, fibrils, and neurodegeneration: Separating the responsible protein aggregates from the innocent bystanders. Annu Rev Neurosci. 2003;26:267–298. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

