The ClpP protease of Streptococcus pneumoniae modulates virulence gene expression and protects against fatal pneumococcal challenge
- PMID: 15385462
- PMCID: PMC517602
- DOI: 10.1128/IAI.72.10.5646-5653.2004
The ClpP protease of Streptococcus pneumoniae modulates virulence gene expression and protects against fatal pneumococcal challenge
Abstract
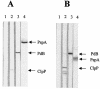
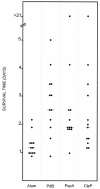
Streptococcus pneumoniae usually colonizes the nasopharynx of humans asymptomatically but occasionally translocates from this niche to the lungs, the brain, and the blood, causing potentially fatal infections. Spread to other host tissues requires a significant morphological change and the expression of virulence factors, such as capsular polysaccharide, and virulence proteins, such as pneumolysin (Ply), PspA, and CbpA. Modulation of the expression of pneumococcal virulence genes by heat shock and by heat shock proteins ClpL and ClpP, as well as the attenuation of virulence of a clpP mutant in a murine intraperitoneal infection model, was demonstrated previously. In this study, we further investigated the underlying mechanism of virulence attenuation by the clpP mutation. The half-lives of the mRNAs of ply and of the first gene of the serotype 2 capsule synthesis locus [cps2A] in the clpP mutant were more than twofold longer than those of the parent after heat shock, suggesting that the mRNA species were regulated posttranscriptionally by ClpP. In addition, the clpP mutant was defective in colonization of the nasopharynx and survival in the lungs of mice after intranasal challenge. The mutant was also killed faster than the parent in the murine macrophage RAW264.7 cell line, indicating that ClpP is required for colonization and intracellular survival in the host. Furthermore, fractionation studies demonstrated that ClpP was translocated into the cell wall after heat shock, and immunization of mice with ClpP elicited a protective immune response against fatal systemic challenge with S. pneumoniae D39, making ClpP a potential vaccine candidate for pneumococcal disease.
Figures




Similar articles
-
Effect of heat shock and mutations in ClpL and ClpP on virulence gene expression in Streptococcus pneumoniae.Infect Immun. 2003 Jul;71(7):3757-65. doi: 10.1128/IAI.71.7.3757-3765.2003. Infect Immun. 2003. PMID: 12819057 Free PMC article.
-
Global transcriptional analysis of clpP mutations of type 2 Streptococcus pneumoniae and their effects on physiology and virulence.J Bacteriol. 2002 Jul;184(13):3508-20. doi: 10.1128/JB.184.13.3508-3520.2002. J Bacteriol. 2002. PMID: 12057945 Free PMC article.
-
Differential expression of key pneumococcal virulence genes in vivo.Microbiology (Reading). 2006 Feb;152(Pt 2):305-311. doi: 10.1099/mic.0.28438-0. Microbiology (Reading). 2006. PMID: 16436418
-
The role of Streptococcus pneumoniae virulence factors in host respiratory colonization and disease.Nat Rev Microbiol. 2008 Apr;6(4):288-301. doi: 10.1038/nrmicro1871. Nat Rev Microbiol. 2008. PMID: 18340341 Review.
-
Emerging concepts in the pathogenesis of the Streptococcus pneumoniae: From nasopharyngeal colonizer to intracellular pathogen.Cell Microbiol. 2019 Nov;21(11):e13077. doi: 10.1111/cmi.13077. Epub 2019 Jul 17. Cell Microbiol. 2019. PMID: 31251447 Free PMC article. Review.
Cited by
-
Variability of Clostridium difficile surface proteins and specific serum antibody response in patients with Clostridium difficile-associated disease.J Clin Microbiol. 2005 Oct;43(10):5018-25. doi: 10.1128/JCM.43.10.5018-5025.2005. J Clin Microbiol. 2005. PMID: 16207956 Free PMC article.
-
Immunization with a combination of three pneumococcal proteins confers additive and broad protection against Streptococcus pneumoniae Infections in Mice.Infect Immun. 2010 Mar;78(3):1276-83. doi: 10.1128/IAI.00473-09. Epub 2009 Dec 28. Infect Immun. 2010. PMID: 20038538 Free PMC article.
-
Bacterial proteases, untapped antimicrobial drug targets.J Antibiot (Tokyo). 2017 Apr;70(4):366-377. doi: 10.1038/ja.2016.138. Epub 2016 Nov 30. J Antibiot (Tokyo). 2017. PMID: 27899793 Review.
-
Proteomic Characterization of Virulence Factors and Related Proteins in Enterococcus Strains from Dairy and Fermented Food Products.Int J Mol Sci. 2022 Sep 19;23(18):10971. doi: 10.3390/ijms231810971. Int J Mol Sci. 2022. PMID: 36142880 Free PMC article.
-
Identification of SP1683 as a pneumococcal protein that is protective against nasopharyngeal colonization.Hum Vaccin Immunother. 2018 May 4;14(5):1234-1242. doi: 10.1080/21645515.2018.1430541. Epub 2018 Feb 21. Hum Vaccin Immunother. 2018. PMID: 29400602 Free PMC article.
References
-
- Beachey, E. H., C. S. Giampapa, and S. N. Abraham. 1988. Bacterial adherence. Adhesin receptor-mediated attachment of pathogenic bacteria to mucosal surfaces. Am. Rev. Respir. Dis. 138:S45-S48. - PubMed
-
- Buchmeier, N. A., and F. Heffron. 1990. Induction of Salmonella stress proteins upon infection of macrophages. Science 248:730-732. - PubMed
-
- Charpentier, E., R. Novak, and E. Tuomanen. 2000. Regulation of growth inhibition at high temperature, autolysis, transformation and adherence in Streptococcus pneumoniae by clpC. Mol. Microbiol. 37:717-726. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

