Release of periplasmic proteins of Brucella suis upon acidic shock involves the outer membrane protein Omp25
- PMID: 15385468
- PMCID: PMC517528
- DOI: 10.1128/IAI.72.10.5693-5703.2004
Release of periplasmic proteins of Brucella suis upon acidic shock involves the outer membrane protein Omp25
Abstract
The survival and replication of Brucella in macrophages is initially triggered by a low intraphagosomal pH. In order to identify proteins released by Brucella during this early acidification step, we analyzed Brucella suis conditioned medium at various pH levels. No significant proteins were released at pH 4.0 in minimal medium or citrate buffer, whereas in acetate buffer, B. suis released a substantial amount of soluble proteins. Comparison of 13 N-terminal amino acid sequences determined by Edman degradation with their corresponding genomic sequences revealed that all of these proteins possessed a signal peptide indicative of their periplasmic location. Ten proteins are putative substrate binding proteins, including a homologue of the nopaline binding protein of Agrobacterium tumefaciens. The absence of this homologue in Brucella melitensis was due to the deletion of a 7.7-kb DNA fragment in its genome. We also characterized for the first time a hypothetical 9.8-kDa basic protein composed of five amino acid repeats. In B. suis, this protein contained 9 repeats, while 12 were present in the B. melitensis orthologue. B. suis in acetate buffer depended on neither the virB type IV secretory system nor the omp31 gene product. However, the integrity of the omp25 gene was required for release at acidic pH, while the absence of omp25b or omp25c displayed smaller effects. Together, these results suggest that Omp25 is involved in the membrane permeability of Brucella in acidic medium.
Figures
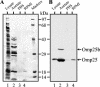







References
-
- Al Dahouk, S., H. Tomaso, K. Nockler, H. Neubauer, and D. Frangoulidis. 2003. Laboratory-based diagnosis of brucellosis—a review of the literature. Part I. Techniques for direct detection and identification of Brucella spp. Clin. Lab. 49:487-505. - PubMed
-
- Al Dahouk, S., H. Tomaso, K. Nockler, H. Neubauer, and D. Frangoulidis. 2003. Laboratory-based diagnosis of brucellosis—a review of the literature. Part II. Serological tests for brucellosis. Clin. Lab. 49:577-589. - PubMed
-
- Alvarez-Martinez, M.-T., J. Machold, C. Weise, H. Schmidt-Eisenlohr, C. Baron, and B. Rouot. 2001. The Brucella suis homologue of the Agrobacterium tumefaciens chromosomal virulence operon chvE is essential for sugar utilization but not for survival in macrophages. J. Bacteriol. 183:5343-5351. - PMC - PubMed
-
- Ausubel, S. F., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seichman, J. A. Smith, and K. Struhl. 1989. Current protocols in molecular biology. John Wiley & Sons, New York, N.Y.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

