Comparison of immunogenicities of recombinant Plasmodium vivax merozoite surface protein 1 19- and 42-kiloDalton fragments expressed in Escherichia coli
- PMID: 15385477
- PMCID: PMC517592
- DOI: 10.1128/IAI.72.10.5775-5782.2004
Comparison of immunogenicities of recombinant Plasmodium vivax merozoite surface protein 1 19- and 42-kiloDalton fragments expressed in Escherichia coli
Abstract
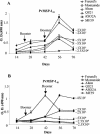
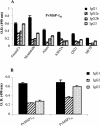
The 42- and 19-kDa C-terminal fragments of merozoite surface protein 1 (MSP-1(42) and MSP-1(19), respectively) are both promising blood-stage vaccine candidate antigens. At present, it is not clear which of the two antigens will be more suitable for inclusion in a cocktail malaria vaccine. In the present study, we expressed the two C-terminal fragments of Plasmodium vivax MSP-1 (PvMSP-1) in an Escherichia coli expression system and purified them by using a rapid two-step protocol. Both of the products were recognized by monoclonal antibodies against PvMSP-1 as well as by immune sera from several individuals exposed to P. vivax. We analyzed and compared the immunological responses to recombinant PvMSP-1(19) and PvMSP-1(42) in mice by using six different adjuvant formulations. Moderate to high antibody responses were observed with both of the antigens in different adjuvant formulations. Surprisingly, alum, which is generally considered to be a poor adjuvant for recombinant malaria antigens, was found to be as good an adjuvant as Montanide ISA 720, ASO2A, and other adjuvant formulations. Most adjuvant formulations induced high levels of immunoglobulin G1 (IgG1), followed by IgG3 and IgG2. Lymphocytes from animals in the PvMSP-1(42)- and PvMSP-1(19)-immunized groups showed proliferative responses upon stimulation with the respective antigens, and high levels of interleukin-4 (IL-4), IL-5, and gamma interferon were detected in the culture supernatants. Immunodepletion studies with sera from mice immunized with these two antigens showed that while immunization with PvMSP-1(42) does produce a PvMSP-1(19)-specific response, a substantial portion is also focused on structures in PvMSP-1(42) not represented by the epidermal growth factor-like domains of PvMSP-1(19). These findings may have implications for the design of MSP-1-based vaccine constructs.
Figures



Similar articles
-
Purification, characterization, and immunogenicity of a disulfide cross-linked Plasmodium vivax vaccine candidate antigen, merozoite surface protein 1, expressed in Escherichia coli.Infect Immun. 2001 Sep;69(9):5464-70. doi: 10.1128/IAI.69.9.5464-5470.2001. Infect Immun. 2001. PMID: 11500418 Free PMC article.
-
Biochemical and immunological characterization of E. coli expressed 42 kDa fragment of Plasmodium vivax and P. cynomolgi bastianelli merozoite surface protein-1.Indian J Biochem Biophys. 2007 Dec;44(6):429-36. Indian J Biochem Biophys. 2007. PMID: 18320841
-
Antigenicity and immunogenicity of Plasmodium vivax merozoite surface protein-3.PLoS One. 2013;8(2):e56061. doi: 10.1371/journal.pone.0056061. Epub 2013 Feb 14. PLoS One. 2013. PMID: 23457498 Free PMC article.
-
Vaccination for vivax malaria: targeting the invaders.Trends Parasitol. 2004 Mar;20(3):99-102. doi: 10.1016/j.pt.2003.12.003. Trends Parasitol. 2004. PMID: 16676415 Review.
-
N-terminal Plasmodium vivax merozoite surface protein-1, a potential subunit for malaria vivax vaccine.Clin Dev Immunol. 2013;2013:965841. doi: 10.1155/2013/965841. Epub 2013 Sep 28. Clin Dev Immunol. 2013. PMID: 24187566 Free PMC article. Review.
Cited by
-
High-Level Expression of Immunogenic Recombinant Plasmodium vivax Merozoite Surface Protein (Pvmsp-142 kDa) in pGEX 6P1 Vector.Iran J Public Health. 2015 Jan;44(1):89-99. Iran J Public Health. 2015. PMID: 26060780 Free PMC article.
-
Genetic diversity and natural selection of Plasmodium vivax reticulocyte invasion genes in Ecuador.Malar J. 2023 Aug 3;22(1):225. doi: 10.1186/s12936-023-04640-0. Malar J. 2023. PMID: 37537581 Free PMC article.
-
Comparative Immunogenicities of full-length Plasmodium falciparum merozoite surface protein 3 and a 24-kilodalton N-terminal fragment.Clin Vaccine Immunol. 2011 Aug;18(8):1221-8. doi: 10.1128/CVI.00064-11. Epub 2011 Jun 1. Clin Vaccine Immunol. 2011. PMID: 21632889 Free PMC article.
-
Immunogenicity of bacterial-expressed recombinant Plasmodium knowlesi merozoite surface protein-142 (MSP-142).Malar J. 2013 Dec 19;12:454. doi: 10.1186/1475-2875-12-454. Malar J. 2013. PMID: 24354660 Free PMC article.
-
Genetic polymorphism and natural selection in the C-terminal 42 kDa region of merozoite surface protein-1 among Plasmodium vivax Korean isolates.Malar J. 2012 Jun 18;11:206. doi: 10.1186/1475-2875-11-206. Malar J. 2012. PMID: 22709605 Free PMC article.
References
-
- Aguado, T., H. Engers, T. Pang, and R. Pink. 1999. Novel adjuvants currently in clinical testing November 2-4, 1998, Fondation Merieux, Annecy, France: a meeting sponsored by the World Health Organization. Vaccine 17:2321-2328. - PubMed
-
- Ahlborg, N., I. T. Ling, A. A. Holder, and E. M. Riley. 2000. Linkage of exogenous T-cell epitopes to the 19-kilodalton region of Plasmodium yoelii merozoite surface protein 1 (MSP-119) can enhance protective immunity against malaria and modulate the immunoglobulin subclass response to MSP-119. Infect. Immun. 68:2102-2109. - PMC - PubMed
-
- Ahlborg, N., I. T. Ling, W. Howard, A. A. Holder, and E. M. Riley. 2002. Protective immune responses to the 42-kilodalton (kDa) region of Plasmodium yoelii merozoite surface protein 1 are induced by the C-terminal 19-kDa region but not by the adjacent 33-kDa region. Infect. Immun. 70:820-825. - PMC - PubMed
-
- Beck, H. P., I. Felger, B. Genton, N. Alexander, F. Al-Yaman, R. F. Anders, and M. Alpers. 1995. Humoral and cell-mediated immunity to the Plasmodium falciparum ring-infected erythrocyte surface antigen in an adult population exposed to highly endemic malaria. Infect. Immun. 63:596-600. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

